Slide 1 Periodic Trends Slide 2 New Vocabulary

Slide 1 Periodic Trends

Slide 2 New Vocabulary l. Electron Shielding The reduction of the attraction between a positively charged nucleus and its outermost electrons due to the cancellation of a portion of the positive charge by the innermost electrons

Slide 3 Atomic Radii Down a Group –Atomic radii generally increase as you move down a group –This trend is caused by the addition of an energy level from one row to the next

Slide 4 Atomic Radii Across a Period –Atomic radii generally decrease as you move across a period –This trend is caused by the increasing positive charge of the nucleus as protons are added
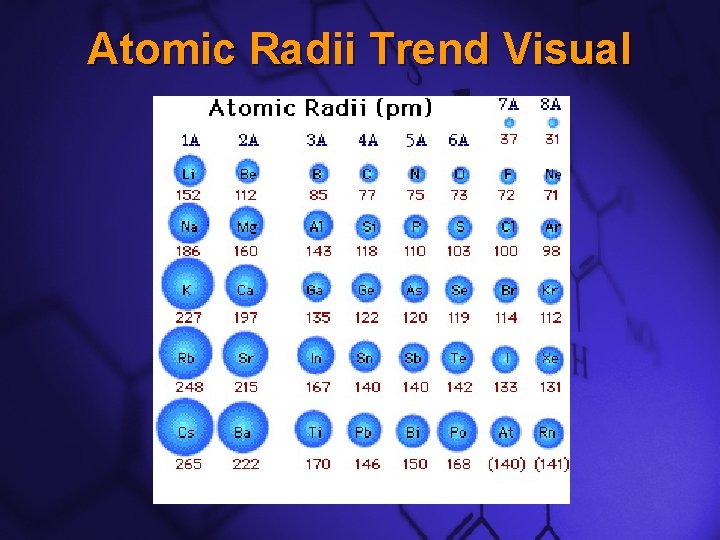
Slide 5 Atomic Radii Trend Visual

Slide 6 Review Vocabulary l. Ionization Energy The amount of energy needed to remove an outer electron from an atom in its ground state and in gas phase

Slide 7 Ionization Energy Down a Group –This trend is the opposite of atomic radii. –The closer or more tightly bound an electron is to its nucleus, the harder it will be to remove –As you move down a group, ionization energy decreases because the electrons are further from their nucleus

Slide 8 Ionization Energy Across a Period –This trend is also the opposite of atomic radii –As you move across a period, ionization energy increases because the electrons are closer to their nucleus.
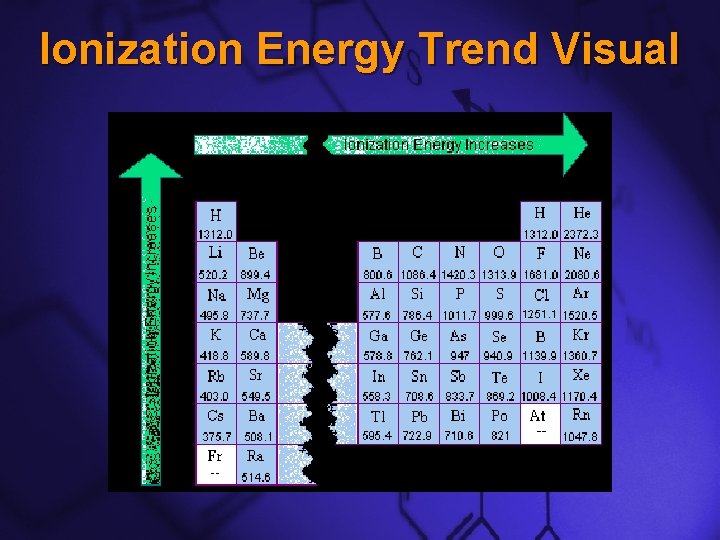
Slide 9 Ionization Energy Trend Visual
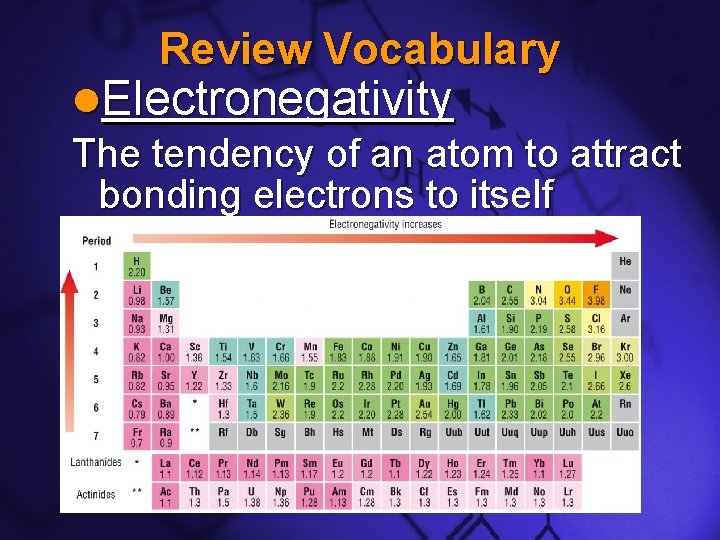
Slide 10 Review Vocabulary l. Electronegativity The tendency of an atom to attract bonding electrons to itself
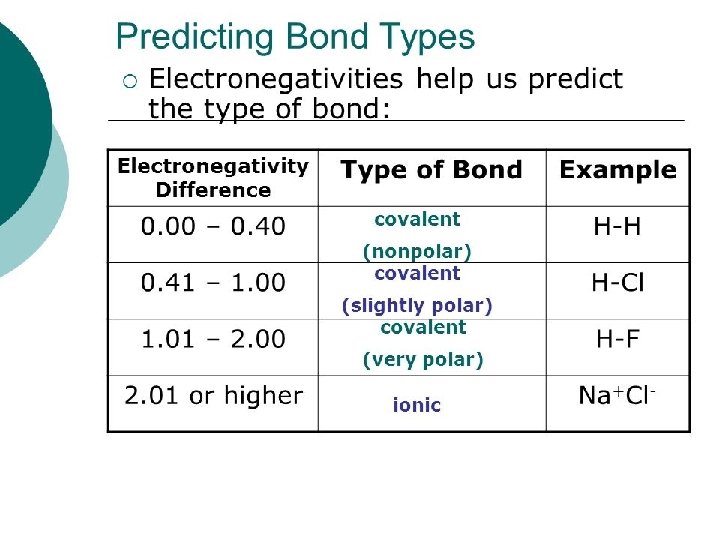
Slide 11 Review Vocabulary l. Electronegativity The tendency of an atom to attract bonding electrons to itself

Slide 12 Electronegativity Down a Group –This trend is the opposite of atomic radii. –Electronegativities generally decrease as you move down a group.

Slide 13 Electronegativity Across a Period –This trend is also the opposite of atomic radii. –Electronegativities generally increase as you move across a period. –This is because the Alkali Metals want to give away their single valence electron so that they will have the same electron configuration as a Noble Gas, S 2 P 6.

Slide 14 Electronegativity Across a Period Continued… –However, Halogens need just one electron to become like Noble Gases. –Therefore, the Halogens’ tendency to attract bonding electrons is very high.
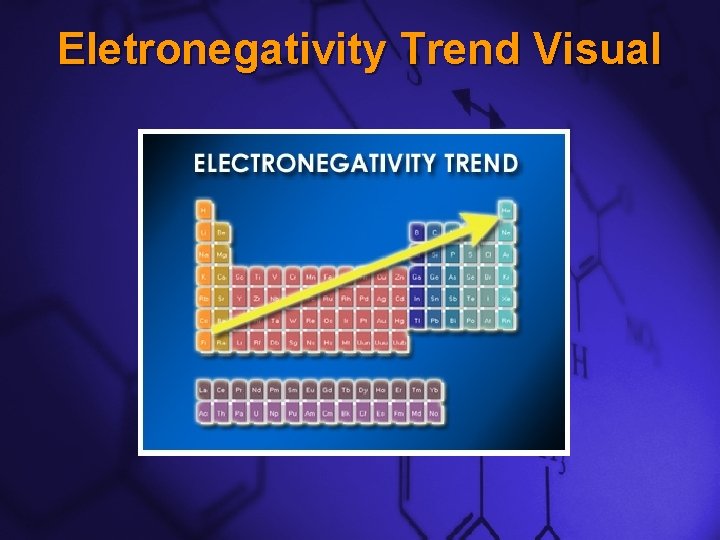
Slide 15 Eletronegativity Trend Visual
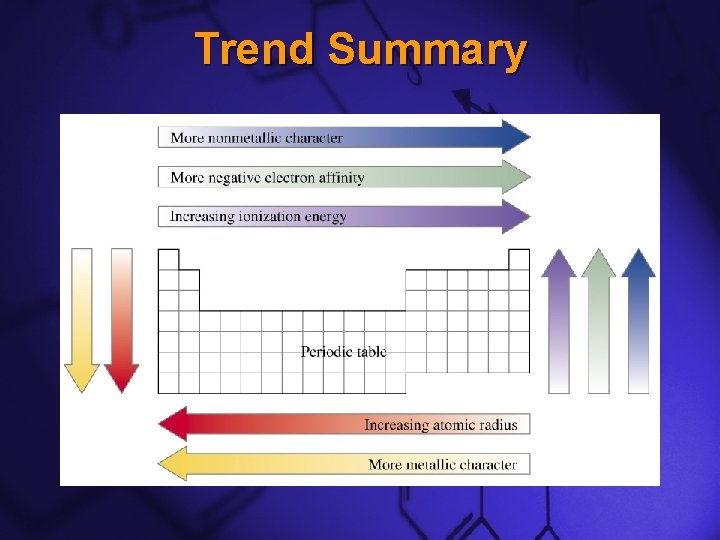
Slide 20 Trend Summary
- Slides: 16