Skin Sensitization and AOP Quanshun Zhang and Erin








- Slides: 32

Skin Sensitization and AOP Quanshun Zhang and Erin Hill Institute for In Vitro Sciences, Inc. 30 West Watkins Mill Road Gaithersburg, Maryland 20878 USA www. iivs. org

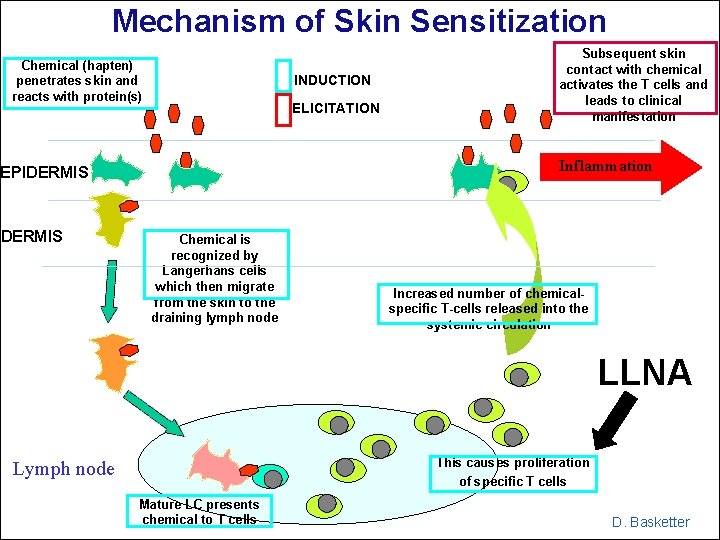
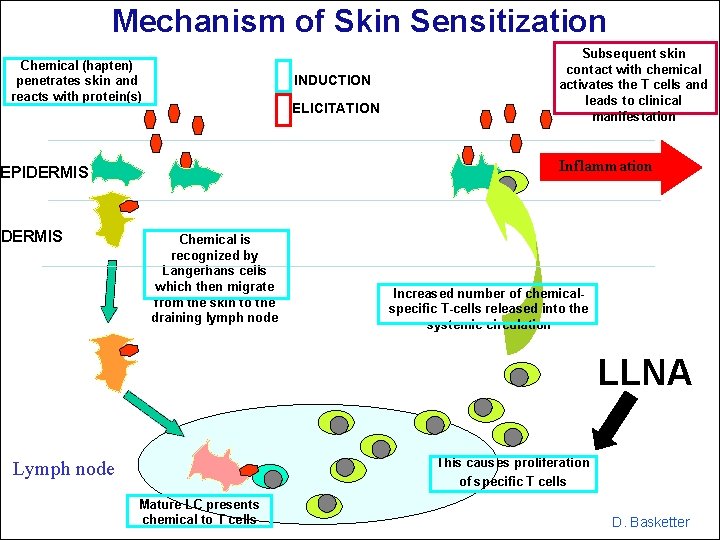
Mechanism of Skin Sensitization Chemical (hapten) penetrates skin and reacts with protein(s) INDUCTION ELICITATION Inflammation EPIDERMIS Subsequent skin contact with chemical activates the T cells and leads to clinical manifestation Chemical is recognized by Langerhans cells which then migrate from the skin to the draining lymph node Increased number of chemicalspecific T-cells released into the systemic circulation LLNA This causes proliferation of specific T cells Lymph node Mature LC presents chemical to T cells D. Basketter

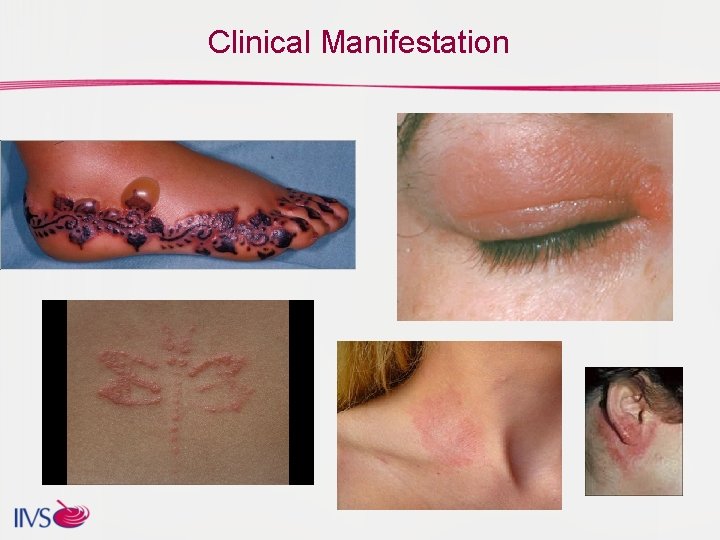
Clinical Manifestation

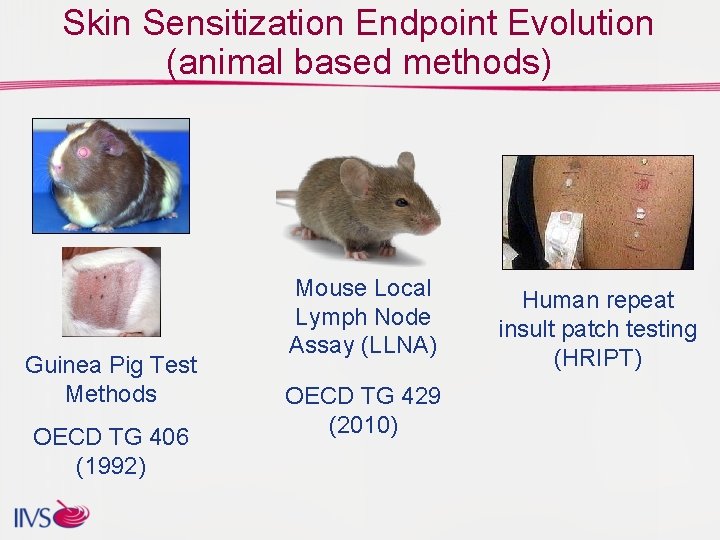
Skin Sensitization Endpoint Evolution (animal based methods) Guinea Pig Test Methods OECD TG 406 (1992) Mouse Local Lymph Node Assay (LLNA) OECD TG 429 (2010) Human repeat insult patch testing (HRIPT)

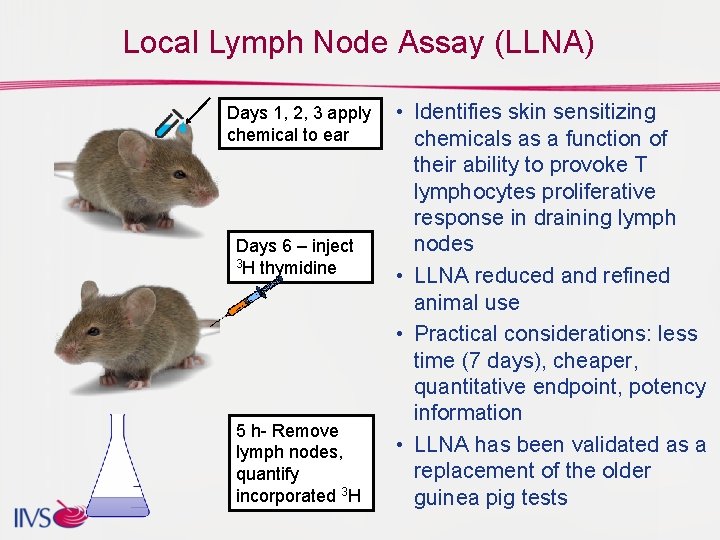
Local Lymph Node Assay (LLNA) Days 1, 2, 3 apply chemical to ear Days 6 – inject 3 H thymidine 5 h- Remove lymph nodes, quantify incorporated 3 H • Identifies skin sensitizing chemicals as a function of their ability to provoke T lymphocytes proliferative response in draining lymph nodes • LLNA reduced and refined animal use • Practical considerations: less time (7 days), cheaper, quantitative endpoint, potency information • LLNA has been validated as a replacement of the older guinea pig tests

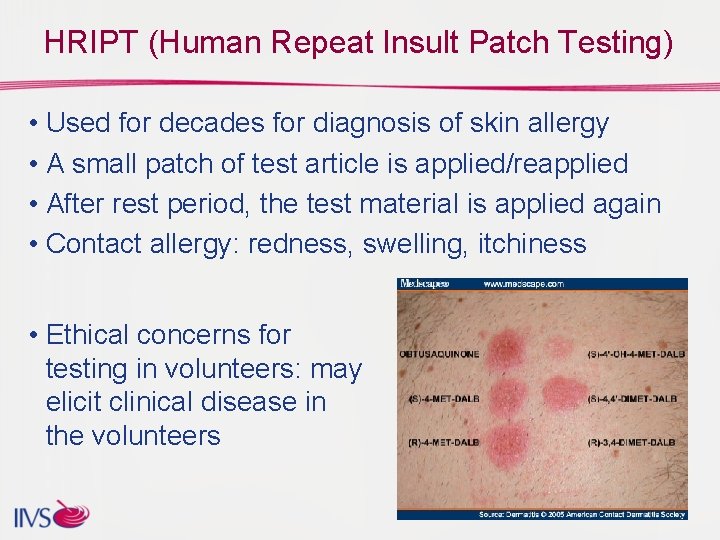
HRIPT (Human Repeat Insult Patch Testing) • Used for decades for diagnosis of skin allergy • A small patch of test article is applied/reapplied • After rest period, the test material is applied again • Contact allergy: redness, swelling, itchiness • Ethical concerns for testing in volunteers: may elicit clinical disease in the volunteers

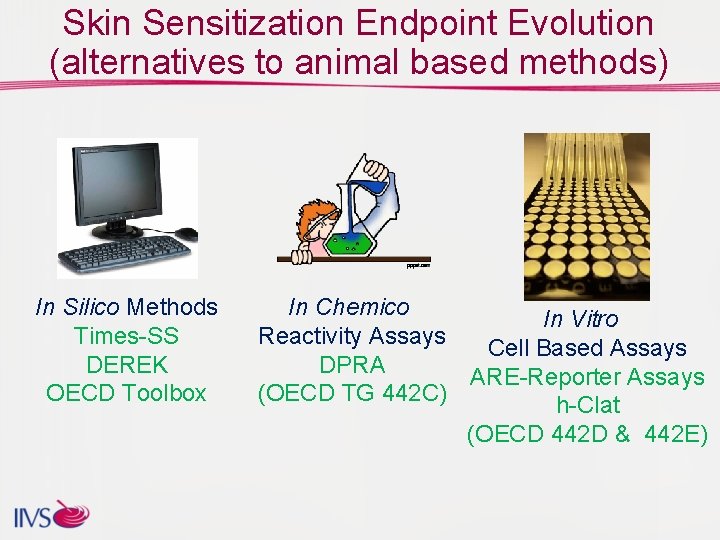
Skin Sensitization Endpoint Evolution (alternatives to animal based methods) In Silico Methods Times-SS DEREK OECD Toolbox In Chemico In Vitro Reactivity Assays Cell Based Assays DPRA ARE-Reporter Assays (OECD TG 442 C) h-Clat (OECD 442 D & 442 E)

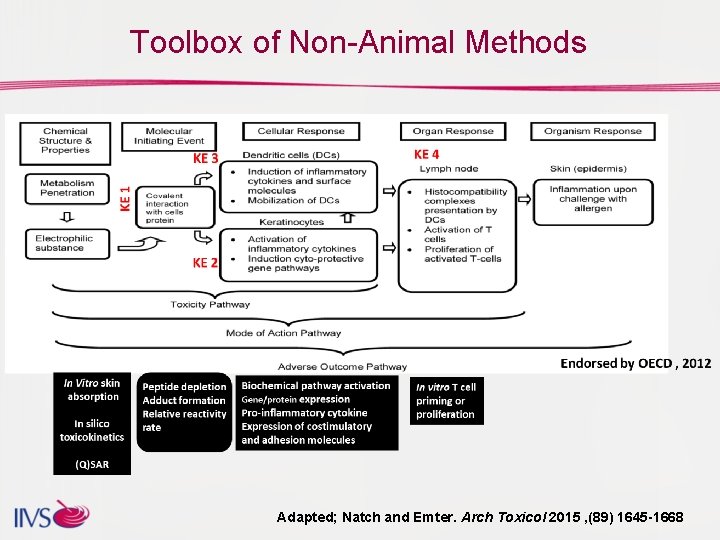
Toolbox of Non-Animal Methods Adapted; Natch and Emter. Arch Toxicol 2015 , (89) 1645 -1668

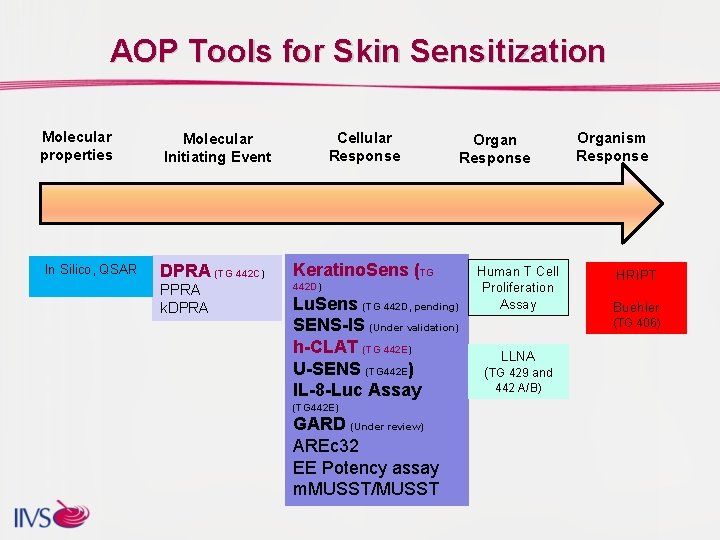
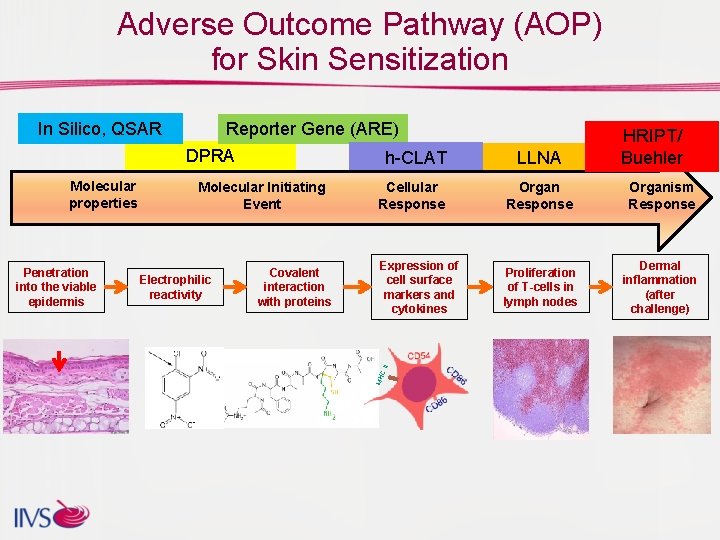
AOP Tools for Skin Sensitization Molecular properties In Silico, QSAR Cellular Response Molecular Initiating Event DPRA (TG 442 C) Keratino. Sens (TG PPRA k. DPRA 442 D) Lu. Sens (TG 442 D, pending) SENS-IS (Under validation) h-CLAT (TG 442 E) U-SENS (TG 442 E) IL-8 -Luc Assay (TG 442 E) GARD (Under review) AREc 32 EE Potency assay m. MUSST/MUSST Organ Response Human T Cell Proliferation Assay Organism Response HRIPT Buehler (TG 406) LLNA (TG 429 and 442 A/B)

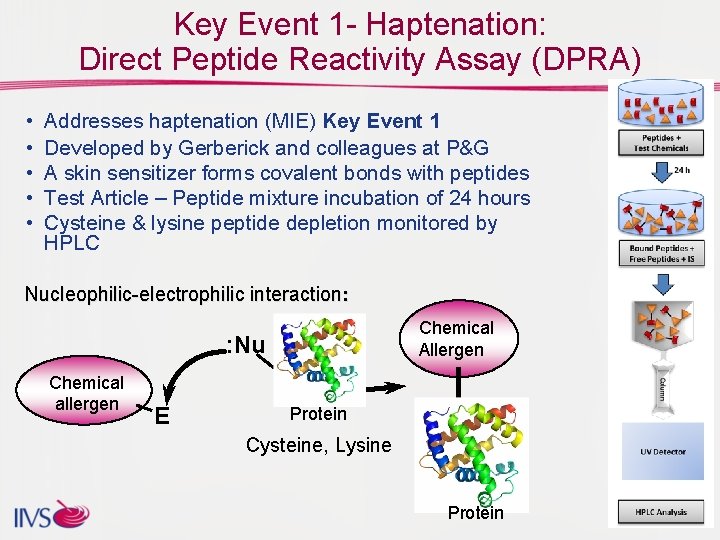
Key Event 1 - Haptenation: Direct Peptide Reactivity Assay (DPRA) • • • Addresses haptenation (MIE) Key Event 1 Developed by Gerberick and colleagues at P&G A skin sensitizer forms covalent bonds with peptides Test Article – Peptide mixture incubation of 24 hours Cysteine & lysine peptide depletion monitored by HPLC Nucleophilic-electrophilic interaction: Chemical Allergen : Nu Chemical allergen E Protein Cysteine, Lysine Protein

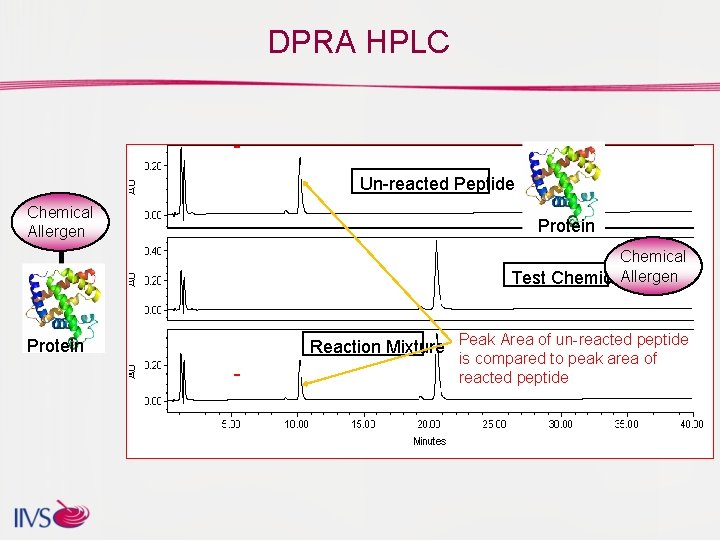
DPRA HPLC Un-reacted Peptide Chemical Allergen Protein Chemical Test Chemical. Allergen Protein Reaction Mixture Peak Area of un-reacted peptide is compared to peak area of reacted peptide

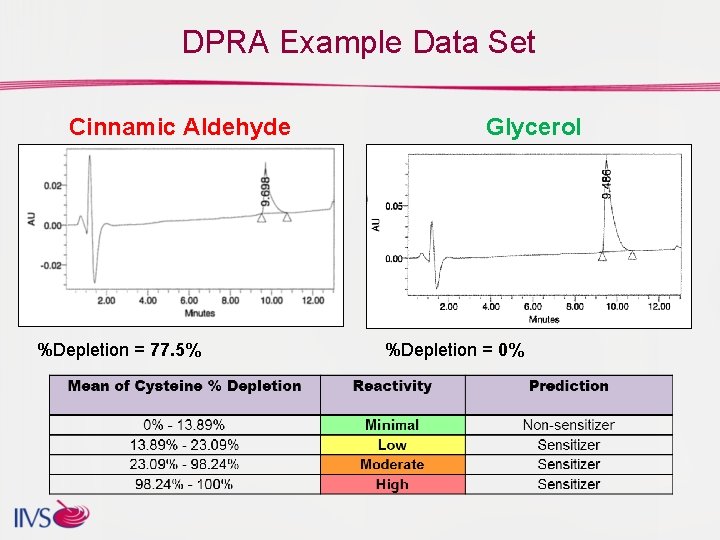
DPRA Example Data Set Cinnamic Aldehyde %Depletion = 77. 5% Glycerol %Depletion = 0%

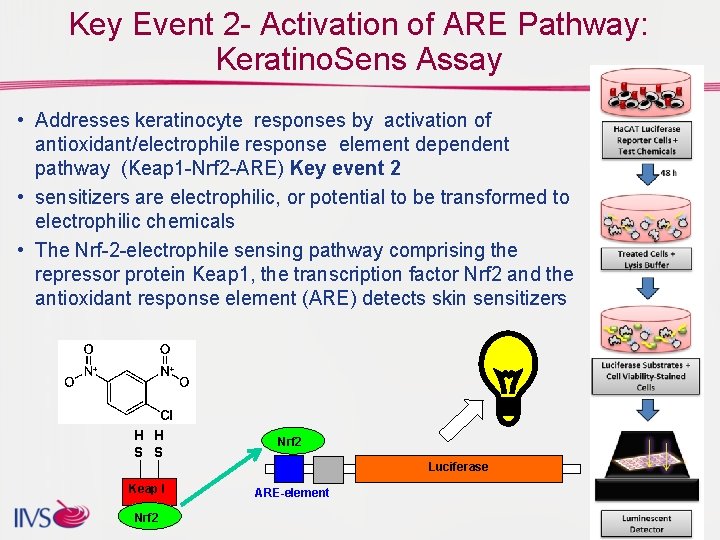
Key Event 2 - Activation of ARE Pathway: Keratino. Sens Assay • Addresses keratinocyte responses by activation of antioxidant/electrophile response element dependent pathway (Keap 1 -Nrf 2 -ARE) Key event 2 • sensitizers are electrophilic, or potential to be transformed to electrophilic chemicals • The Nrf-2 -electrophile sensing pathway comprising the repressor protein Keap 1, the transcription factor Nrf 2 and the antioxidant response element (ARE) detects skin sensitizers H H S S Nrf 2 Luciferase Keap I Nrf 2 ARE-element

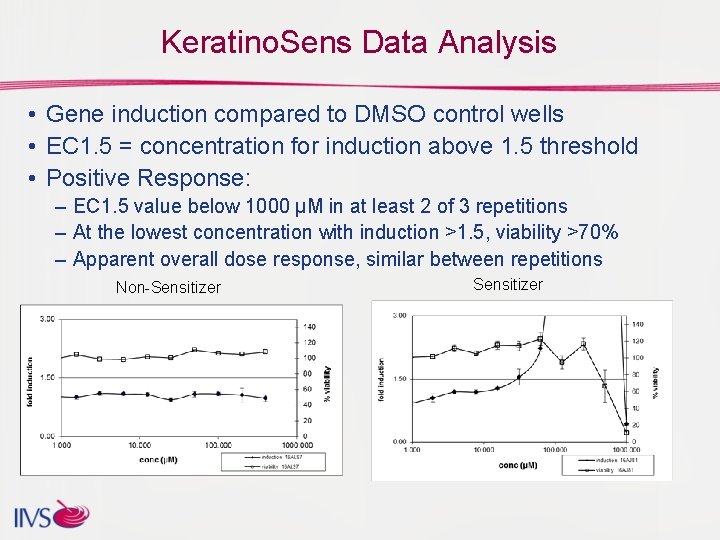
Keratino. Sens Data Analysis • Gene induction compared to DMSO control wells • EC 1. 5 = concentration for induction above 1. 5 threshold • Positive Response: – EC 1. 5 value below 1000 µM in at least 2 of 3 repetitions – At the lowest concentration with induction >1. 5, viability >70% – Apparent overall dose response, similar between repetitions Non-Sensitizer

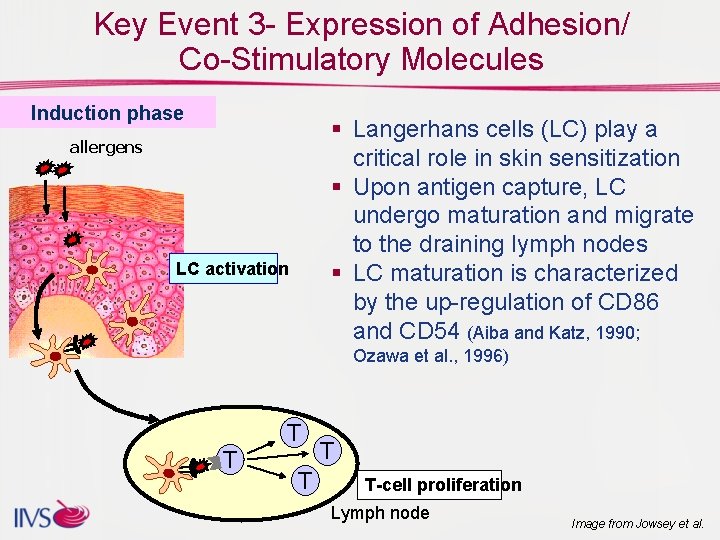
Key Event 3 - Expression of Adhesion/ Co-Stimulatory Molecules Induction phase § Langerhans cells (LC) play a critical role in skin sensitization § Upon antigen capture, LC undergo maturation and migrate to the draining lymph nodes § LC maturation is characterized by the up-regulation of CD 86 and CD 54 (Aiba and Katz, 1990; allergens LC activation Ozawa et al. , 1996) T T T-cell proliferation Lymph node Image from Jowsey et al.

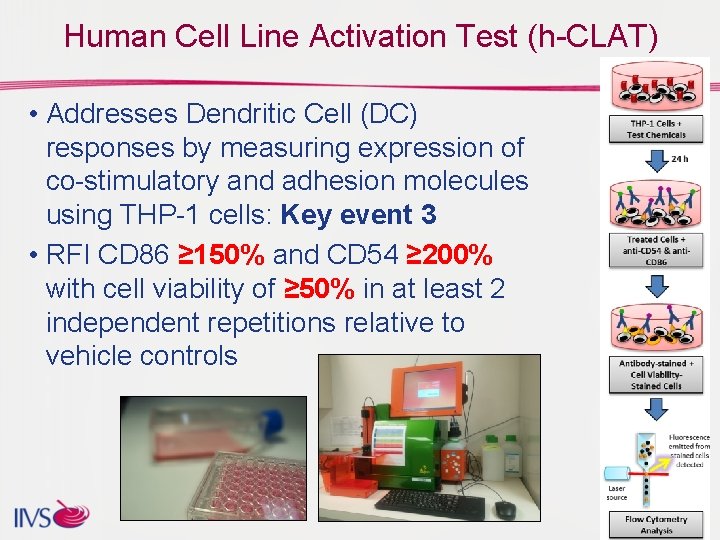
Human Cell Line Activation Test (h-CLAT) • Addresses Dendritic Cell (DC) responses by measuring expression of co-stimulatory and adhesion molecules using THP-1 cells: Key event 3 • RFI CD 86 ≥ 150% and CD 54 ≥ 200% with cell viability of ≥ 50% in at least 2 independent repetitions relative to vehicle controls

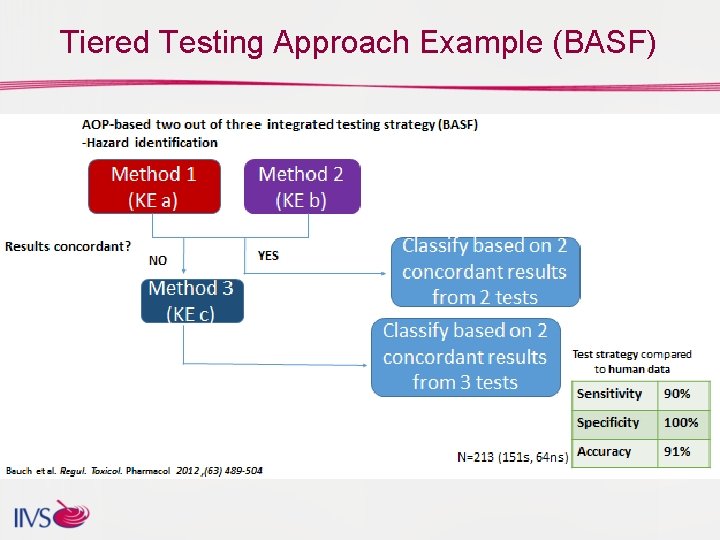
Integrated Testing Strategies (ITS) OECD Test Guidelines for 3 methods (others under review) q Direct Peptide Reactivity Assay (DPRA) (TG 442 C) (2015) q ARE-Nrf 2 Luciferase Method (Keratino. Sens. TM) (TG 442 D) (2015) q Human Cell Line Activation Test (h-CLAT) (TG 442 E) (2016) • OECD- support discrimination between skin sensitizers (UN GHS category 1) and non-sensitizers in combination with other info • Each assay considered for an Integrated Testing Strategy (ITS) for hazard identification; development of Adverse Outcome Pathway (AOP) • Individual assay limitations, integrated approach better • For safety determination, all 3 assays should be used • Test Guidelines cannot be used on their own to sub-categorize skin sensitizers into UN GHS subcategories 1 A and 1 B or to predict potency for safety assessment identification

Mechanism of Skin Sensitization Chemical (hapten) penetrates skin and reacts with protein(s) INDUCTION ELICITATION Inflammation EPIDERMIS Subsequent skin contact with chemical activates the T cells and leads to clinical manifestation Chemical is recognized by Langerhans cells which then migrate from the skin to the draining lymph node Increased number of chemicalspecific T-cells released into the systemic circulation LLNA This causes proliferation of specific T cells Lymph node Mature LC presents chemical to T cells D. Basketter

Adverse Outcome Pathway (AOP) for Skin Sensitization Electrophilic reactivity Covalent interaction with proteins LLNA HRIPT/ Buehler Cellular Response Organism Response Expression of cell surface markers and cytokines Proliferation of T-cells in lymph nodes Dermal inflammation (after challenge) I Penetration into the viable epidermis Molecular Initiating Event CI Molecular properties Reporter Gene (ARE) DPRA h-CLAT MH In Silico, QSAR

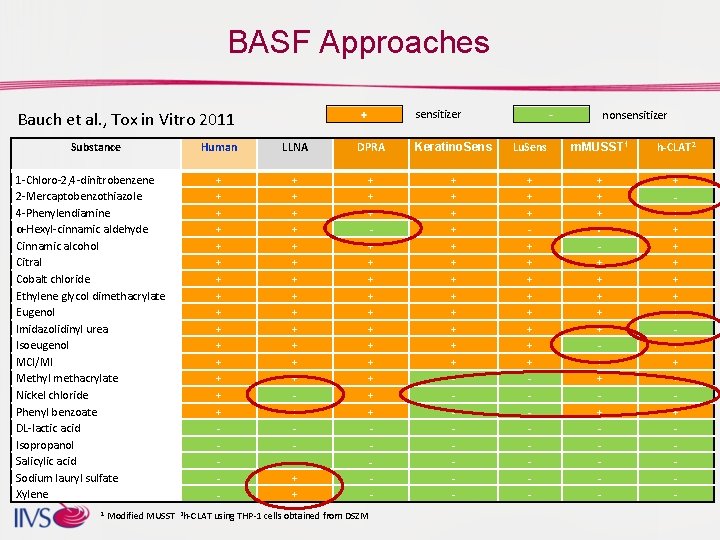
BASF Approaches sensitizer Bauch et al. , Tox in Vitro 2011 Substance 1 -Chloro-2, 4 -dinitrobenzene 2 -Mercaptobenzothiazole 4 -Phenylendiamine α-Hexyl-cinnamic aldehyde Cinnamic alcohol Citral Cobalt chloride Ethylene glycol dimethacrylate Eugenol Imidazolidinyl urea Isoeugenol MCI/MI Methyl methacrylate Nickel chloride Phenyl benzoate DL-lactic acid Isopropanol Salicylic acid Sodium lauryl sulfate Xylene nonsensitizer Human LLNA DPRA Keratino. Sens Lu. Sens m. MUSST 1 h-CLAT 2 + + + + + + + + - + + + + + + + + + + + + - + + + + + + - 1 Modified MUSST 2 h-CLAT using THP-1 cells obtained from DSZM

Tiered Testing Approach Example (BASF)

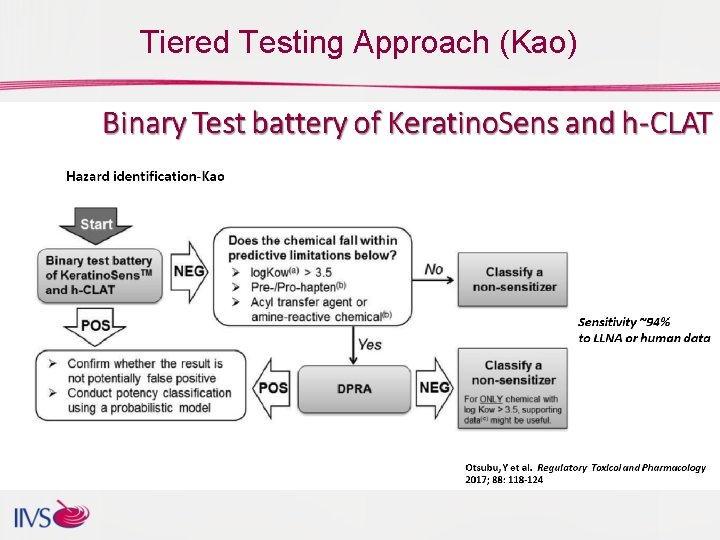
Tiered Testing Approach (Kao)

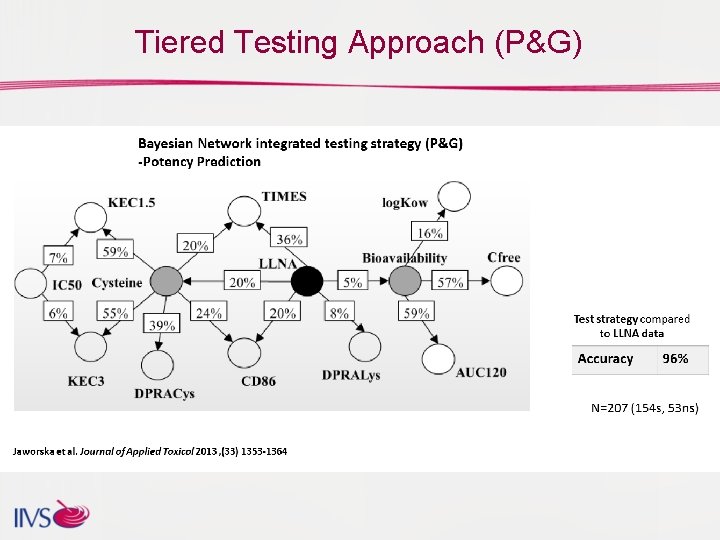
Tiered Testing Approach (P&G)

Botanical Cosmetic Ingredients Could the Keratino. Sens assay detect sensitizing chemicals in a botanical ingredient mixture? – Safety assessment challenging (molar conc. of some components may be unknown) – Cosmetic ingredients containing botanical extracts are formulated with solvents, preservatives, and generally less than 10% extract • We measured the activity of 3 known sensitizers neat and then “spiked” at 1%- 2% into 4 different botanical ingredients (each with a different excipient solvent system) 1. Gluteraldehyde (GA)- strong sensitizer 2. Dimethyl maleate (DM)- moderate sensitizer 3. Cinnamic aldehyde (CA) – moderate sensitizer • Activity of the spiked samples was measured and compared to the neat sensitizer and the unspiked botanical ingredient Study performed in collaboration with

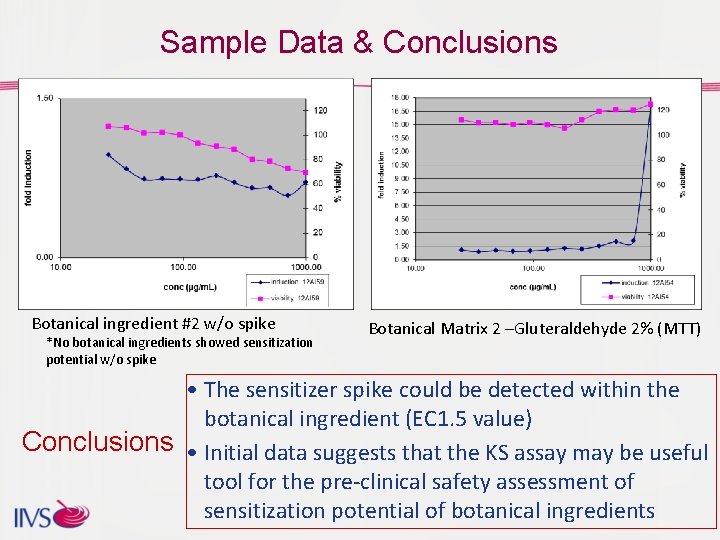
Sample Data & Conclusions Botanical ingredient #2 w/o spike *No botanical ingredients showed sensitization potential w/o spike Botanical Matrix 2 –Gluteraldehyde 2% (MTT) • The sensitizer spike could be detected within the botanical ingredient (EC 1. 5 value) Conclusions • Initial data suggests that the KS assay may be useful tool for the pre-clinical safety assessment of sensitization potential of botanical ingredients

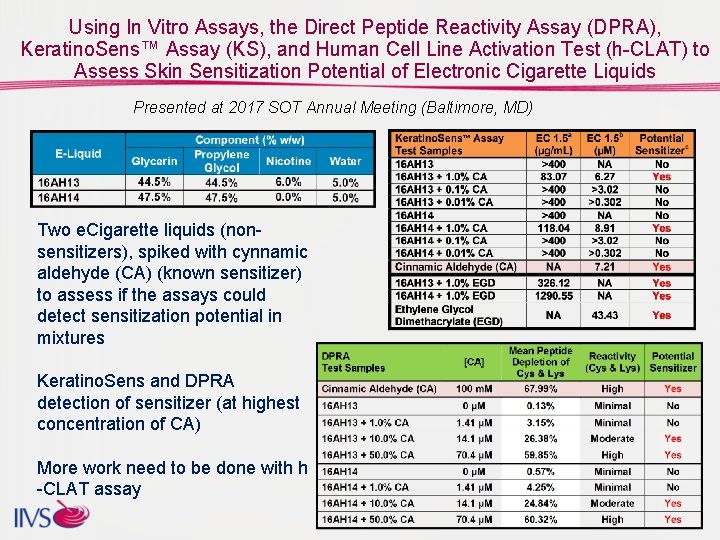
Using In Vitro Assays, the Direct Peptide Reactivity Assay (DPRA), Keratino. Sens™ Assay (KS), and Human Cell Line Activation Test (h-CLAT) to Assess Skin Sensitization Potential of Electronic Cigarette Liquids Presented at 2017 SOT Annual Meeting (Baltimore, MD) Two e. Cigarette liquids (nonsensitizers), spiked with cynnamic aldehyde (CA) (known sensitizer) to assess if the assays could detect sensitization potential in mixtures Keratino. Sens and DPRA detection of sensitizer (at highest concentration of CA) More work need to be done with h -CLAT assay

Considerations for Full Replacement using Available In Vitro, In Chemico, In Silico Methods ØThese assays do not currently address skin penetration & skin metabolism ØSolubility is a concern ØAssessment of Potency ØAvailability/Cost of methods ØBest ITS is one that meets client goals Ø Screening (before clinical), submission? , timing, chemistry, etc. ØSpecial Considerations Ø Mixtures (e. g. Botanicals) Ø Photo-activation Ø Assay Optimizations to address pro- haptens

Recommended References • 1. Non-animal methods to predict skin sensitization (I): the Cosmetics Europe database Http: //doi. org/10. 1080/10408444. 2018. 1429385 • 2. Non-animal methods to predict skin sensitization(II): an assessment of defined approaches Http: //doi. org 10. 1080/10408444. 2018. 1429386

Acknowledgements • • Givaudan P&G S. C. Johnson Mary Kay Merck Keratino. Sens ring trial participants (Givaudan, BASF, Beiersdorf, P&G) Lu. Sens ring trial participants (BASF, P&G, DSM, BRT) The Clorox Company for funds for IIVS participation in Keratino. Sens ring trial • Colgate-Palmolive Company for funds for IIVS participation in Keratino. Sens ring trial • IIVS lab team

Thank you for your attention Questions & Discussion

References Basketter, D. , Alepee, N. , Casati, S. , Crozier, J. , Eigler, D. , Griem, P. , Hubesch, B. , de, K. J. , Landsiedel, R. , Louekari, K. , Manou, I. , Maxwell, G. , Mehling, A. , Netzeva, T. , Petry, T. , Rossi, L. H. , 2013. Skin sensitisation – moving forward with non-animal testing strategies for regulatory purposes in the EU. Regul. Toxicol. Pharmacol. 67, 531– 535. Basketter, D. A. , Alepee, N. , Ashikaga, T. , Barroso, J. , Gilmour, N. , Goebel, C. , Hibatallah, J. , Hoffmann, S. , Kern, P. , Martinozzi-Teissier, S. , Maxwell, G. , Reisinger, K. , Sakaguchi, H. , Schepky, A. , Tailhardat, M. , Templier, M. , 2014. Categorization of chemicals according to their relative human skin sensitizing potency. Dermatitis 25, 11– 21 Bauch et al. (2012) Putting the parts together: Combining in vitro methods to test for skin sensitizing potentials. Reg Tox Pharmacol 63(3): 489 -504 Bauch et al. (2011) Intralaboratory validation of four in vitro assays for the prediction of the skin sensitizing potential of chemicals. Toxicol In Vitro 25(6): 1162 -8. D. Gan, K. Norman, N. Barnes, H. Raabe, C. Gomez, and J. Harbell Application of the Keratino. Sens Assay for Prediction of Dermal Sensitization Hazard for Botanical Cosmetic Ingredients. Presented at 2013 SOT Gerberick, F. , Aleksic, M. , Basketter, D. , Casati, S. , Karlberg, A. T. , Kern, P. , Kimber, I. , Lepoittevin, J. P. , Natsch, A. , Ovigne, J. M. , Rovida, C. , Sakaguchi, H. , Schultz, T. , 2008. Chemical reactivity measurement and the predictive identification of skin sensitisers. Altern. Lab. Anim. 36, 215– 242 Gerberick, G. F. , Ryan, C. A. , Kern, P. , Schlatter, H. , Dearman, R. , Kimber, I. , Patlewicz, G. , Basketter, D. , 2005. Compilation of historical local lymph node data for evalutaion of skin sensitization alternative methods. Dermatitis 16, 157– 202 Gerberick, G. F. , Vassallo, J. D. , Bailey, R. E. , Chaney, J. G. , Morrall, S. W. , Lepoittevin, J. P. , 2004. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 81, 332– 343 Jaworska, JS, Natsch, A, ·Ryan, C, · Strickland, J, Ashikaga, T, · Miyazawa, M. Bayesian integrated testing strategy (ITS) for skin sensitization potency assessment: a decision support system for quantitative weight of evidence and adaptive testing strategy. Arch Toxicol 20 October 2015 DOI 10. 1007/s 00204 -015 -1634 -2

References Miller, et. al. Using In Vitro Assays, the Direct Peptide Reactivity Assay (DPRA), Keratino. Sens™ Assay (KS), and Human Cell Line Activation Test (h-CLAT) to Assess Skin Sensitization Potential of Electronic Cigarette Liquids. Presented at 2017 SOT meeting Natsch A. , Emter R. Reporter cell lines for skin sensitization testing. Arch. Toxicol. 2015; 89: 1645– 1648. doi: 10. 1007/s 00204015 -1555 -0 Natsch A, Ryan CA, Foertsch L, Emter R, Jaworska J, Gerberick F, Kern P. A dataset on 145 chemicals tested in alternative assays for skin sensitization undergoing prevalidation. J Appl Toxicol. 2013 Nov; 33(11): 1337 -52. doi: 10. 1002/jat. 2868. Natsch, A. , Bauch, C. , Foertsch, L. M. , Gerberick, G. F. , Norman, K. , Hilberer, H. , Inglis, H. , Landsiedel, R. , Onken, S. , Reuter, R. , Schepky, A. , Emter, R. , 2011. The intra and inter-laboratory reproducibility and predictivity of the Keratino. Sens assay to predict skin sensitizers in vitro: results of a ring-study in five laboratories. Toxicol. In Vitro 25, 733– 744. OECD 2012 a. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 1: Scientific Evidence. Series on Testing and Assessment No. 168. OECD 2012 b. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 2: Use of the AOP to Develop Chemical Categories and Integrated Assessment and Testing Approaches. Series on Testing and Assessment No. 168. ENV/JM/MONO(2012)10/PART 2 OECD, (2015). Test No. 442 C. In Chemico Skin Sensitization: Direct Peptide Reactivity Assay (DPRA). In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects, OECD Publishing OECD, (2015). Test No. 442 D. In Vitro Skin Sensitisation: ARE-Nrf 2 Luciferase Test Method. In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects, OECD Publishing OECD, (2016). Test No. 442 E. In Vitro Skin Sensitisation: human Cell Line Activation Test (h-CLAT). In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects, OECD Publishing Otsubo, Y, Nishijo, T, Miyazawa, M, Saito, K, Mizumachi, H, Sakaguch, H. Binary test battery with Keratino. Sens™ and h-CLAT as part of a bottom-up approach for skin sensitization hazard prediction. 2017. Regulatory Toxicology and Pharmacology 88 118 -124. Wong, CL, Ghassabian, S, Smith MT, Lam A. In vitro methods for hazard assessment of industrial chemicals–opportunities and challenges. 2015. Frontiers in Pharmacology. doi: 10. 3389/fphar. 2015. 00094