Skeletal Muscle Physiology Susan V Brooks Herzog Department

Skeletal Muscle Physiology Susan V. Brooks Herzog Department of Physiology University of Michigan
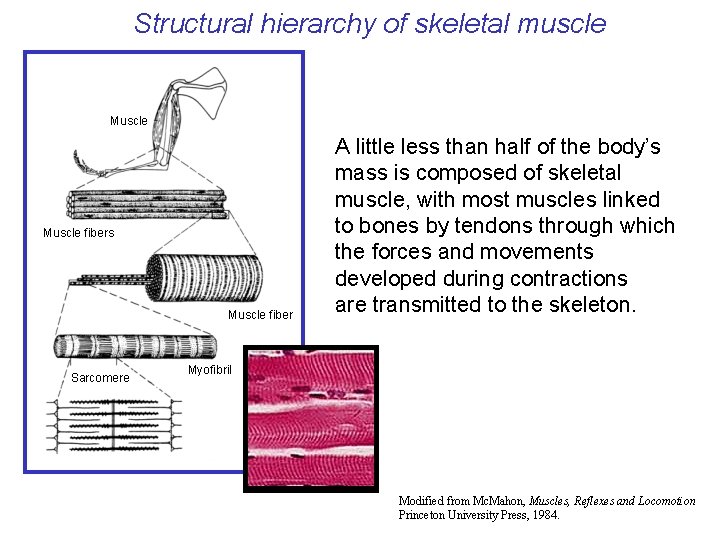
Structural hierarchy of skeletal muscle Muscle fibers Muscle fiber Sarcomere A little less than half of the body’s mass is composed of skeletal muscle, with most muscles linked to bones by tendons through which the forces and movements developed during contractions are transmitted to the skeleton. Myofibril Modified from Mc. Mahon, Muscles, Reflexes and Locomotion Princeton University Press, 1984.
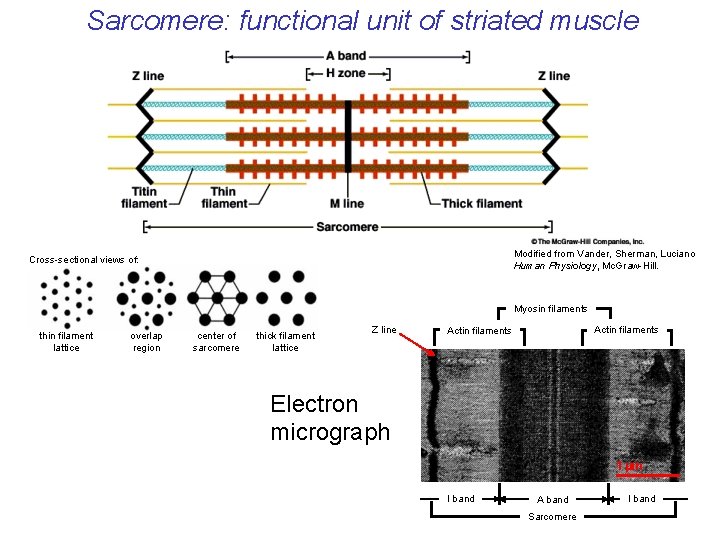
Sarcomere: functional unit of striated muscle Modified from Vander, Sherman, Luciano Human Physiology, Mc. Graw-Hill. Cross-sectional views of: Myosin filaments thin filament lattice overlap region center of sarcomere thick filament lattice Z line Actin filaments Electron micrograph 1 mm I band A band Sarcomere I band
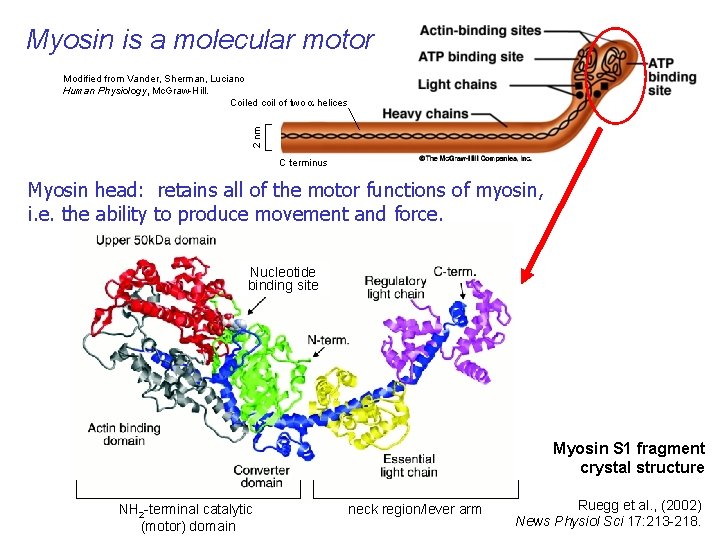
Myosin is a molecular motor 2 nm Modified from Vander, Sherman, Luciano Human Physiology, Mc. Graw-Hill. Coiled coil of two a helices Myosin is a hexamer: 2 myosin heavy chains 4 myosin light chains C terminus Myosin head: retains all of the motor functions of myosin, i. e. the ability to produce movement and force. Nucleotide binding site Myosin S 1 fragment crystal structure NH 2 -terminal catalytic (motor) domain neck region/lever arm Ruegg et al. , (2002) News Physiol Sci 17: 213 -218.
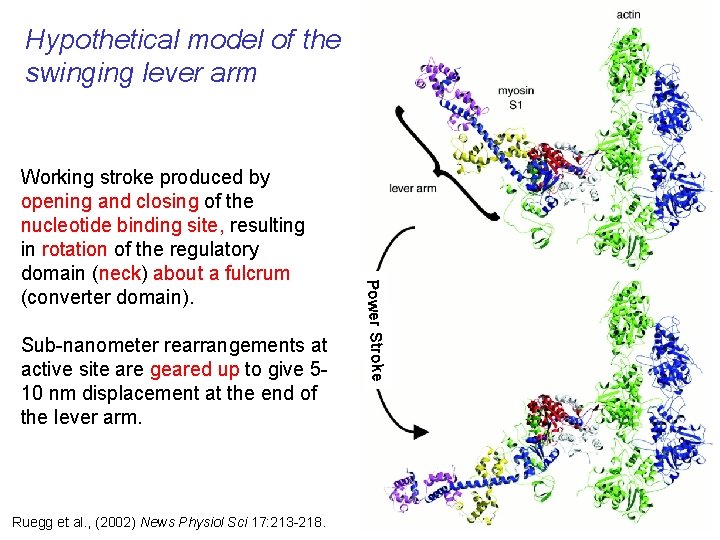
Hypothetical model of the swinging lever arm Sub-nanometer rearrangements at active site are geared up to give 510 nm displacement at the end of the lever arm. Ruegg et al. , (2002) News Physiol Sci 17: 213 -218. Power Stroke Working stroke produced by opening and closing of the nucleotide binding site, resulting in rotation of the regulatory domain (neck) about a fulcrum (converter domain).
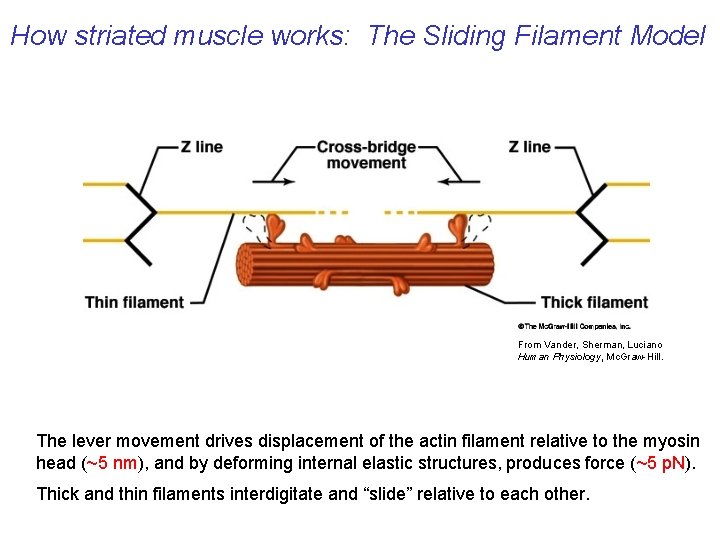
How striated muscle works: The Sliding Filament Model From Vander, Sherman, Luciano Human Physiology, Mc. Graw-Hill. The lever movement drives displacement of the actin filament relative to the myosin head (~5 nm), and by deforming internal elastic structures, produces force (~5 p. N). Thick and thin filaments interdigitate and “slide” relative to each other.
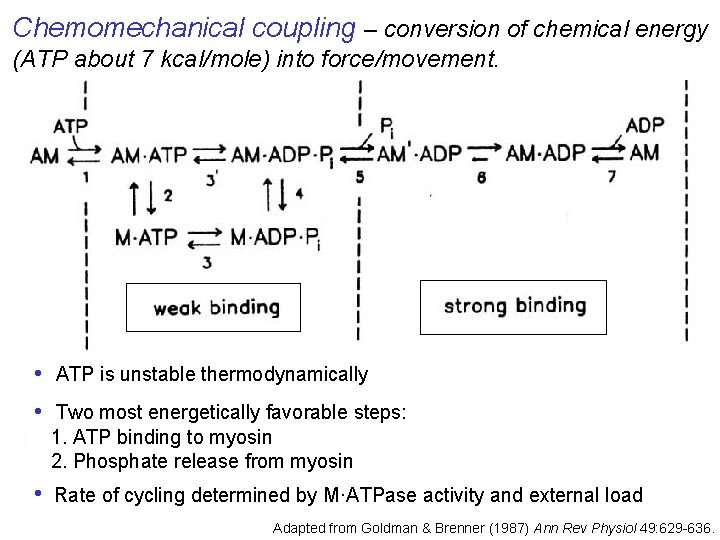
Chemomechanical coupling – conversion of chemical energy (ATP about 7 kcal/mole) into force/movement. • ATP is unstable thermodynamically • Two most energetically favorable steps: 1. ATP binding to myosin 2. Phosphate release from myosin • Rate of cycling determined by M·ATPase activity and external load Adapted from Goldman & Brenner (1987) Ann Rev Physiol 49: 629 -636.
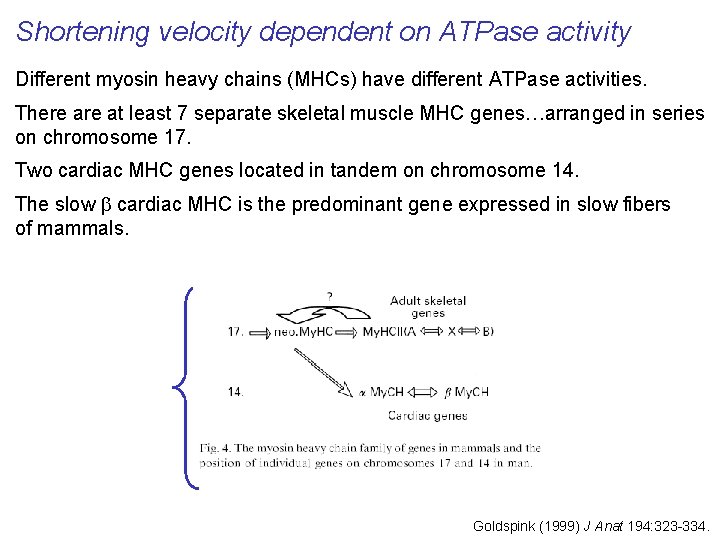
Shortening velocity dependent on ATPase activity Different myosin heavy chains (MHCs) have different ATPase activities. There at least 7 separate skeletal muscle MHC genes…arranged in series on chromosome 17. Two cardiac MHC genes located in tandem on chromosome 14. The slow b cardiac MHC is the predominant gene expressed in slow fibers of mammals. Goldspink (1999) J Anat 194: 323 -334.
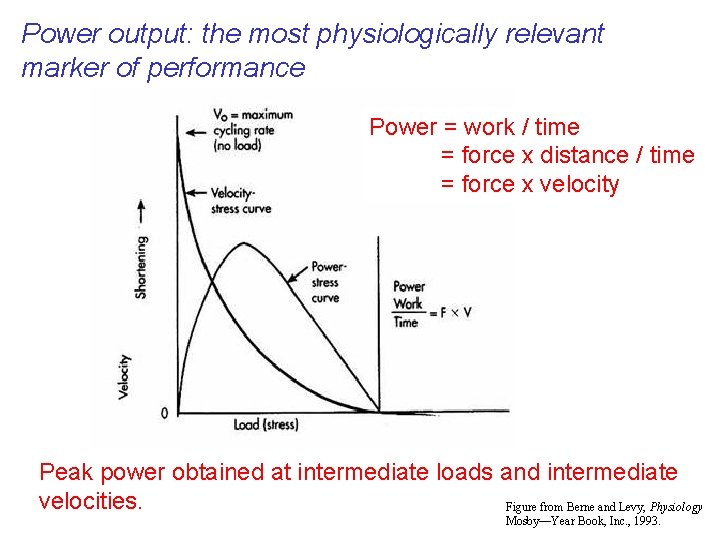
Power output: the most physiologically relevant marker of performance Power = work / time = force x distance / time = force x velocity Peak power obtained at intermediate loads and intermediate velocities. Figure from Berne and Levy, Physiology Mosby—Year Book, Inc. , 1993.
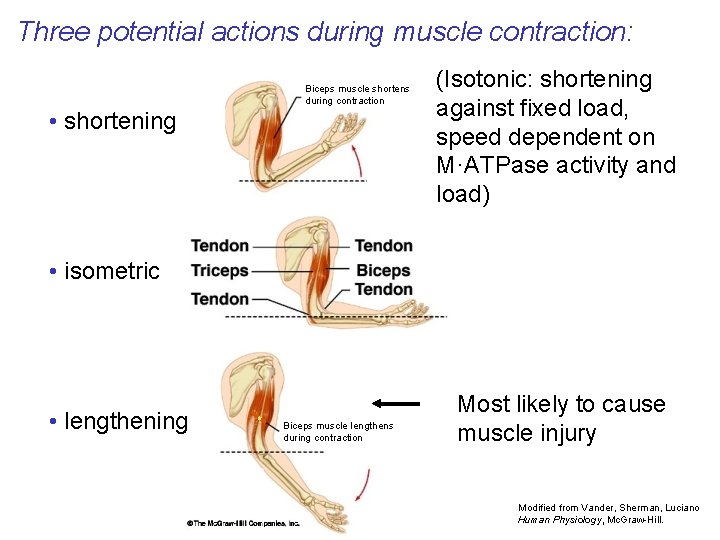
Three potential actions during muscle contraction: Biceps muscle shortens during contraction • shortening (Isotonic: shortening against fixed load, speed dependent on M·ATPase activity and load) • isometric • lengthening Biceps muscle lengthens during contraction Most likely to cause muscle injury Modified from Vander, Sherman, Luciano Human Physiology, Mc. Graw-Hill.
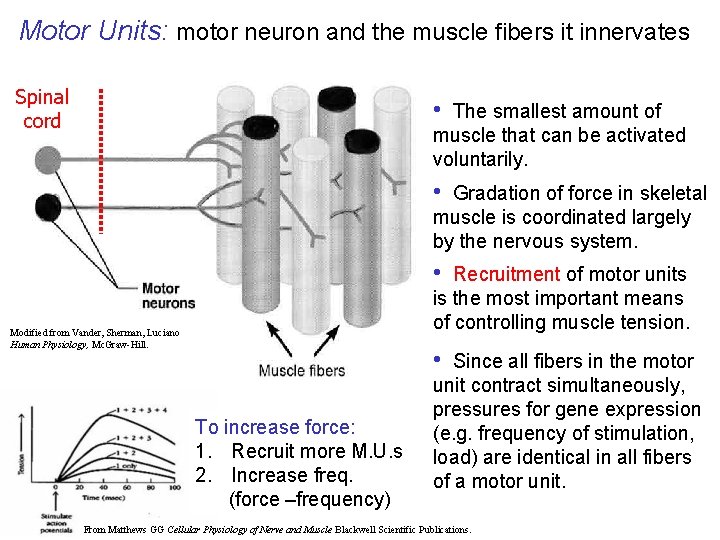
Motor Units: motor neuron and the muscle fibers it innervates Spinal cord • The smallest amount of muscle that can be activated voluntarily. • Gradation of force in skeletal muscle is coordinated largely by the nervous system. • Recruitment of motor units is the most important means of controlling muscle tension. Modified from Vander, Sherman, Luciano Human Physiology, Mc. Graw-Hill. • To increase force: 1. Recruit more M. U. s 2. Increase freq. (force –frequency) Since all fibers in the motor unit contract simultaneously, pressures for gene expression (e. g. frequency of stimulation, load) are identical in all fibers of a motor unit. From Matthews GG Cellular Physiology of Nerve and Muscle Blackwell Scientific Publications.
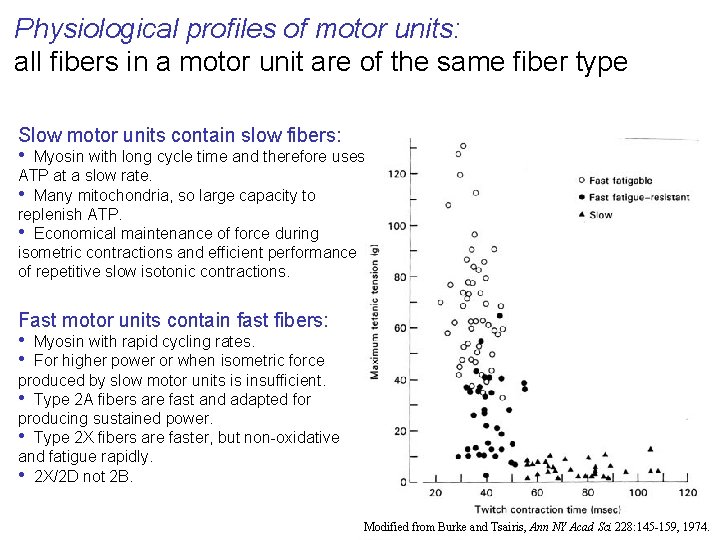
Physiological profiles of motor units: all fibers in a motor unit are of the same fiber type Slow motor units contain slow fibers: • Myosin with long cycle time and therefore uses ATP at a slow rate. • Many mitochondria, so large capacity to replenish ATP. • Economical maintenance of force during isometric contractions and efficient performance of repetitive slow isotonic contractions. Fast motor units contain fast fibers: • Myosin with rapid cycling rates. • For higher power or when isometric force produced by slow motor units is insufficient. • Type 2 A fibers are fast and adapted for producing sustained power. • Type 2 X fibers are faster, but non-oxidative and fatigue rapidly. • 2 X/2 D not 2 B. Modified from Burke and Tsairis, Ann NY Acad Sci 228: 145 -159, 1974.
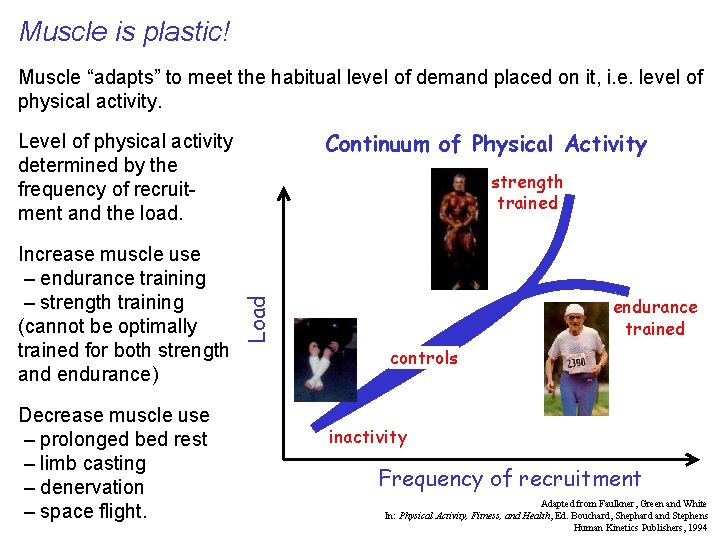
Muscle is plastic! Muscle “adapts” to meet the habitual level of demand placed on it, i. e. level of physical activity. Continuum of Physical Activity Level of physical activity determined by the frequency of recruitment and the load. Decrease muscle use – prolonged bed rest – limb casting – denervation – space flight. endurance trained Load Increase muscle use – endurance training – strength training (cannot be optimally trained for both strength and endurance) strength trained controls inactivity Frequency of recruitment Adapted from Faulkner, Green and White In: Physical Activity, Fitness, and Health, Ed. Bouchard, Shephard and Stephens Human Kinetics Publishers, 1994
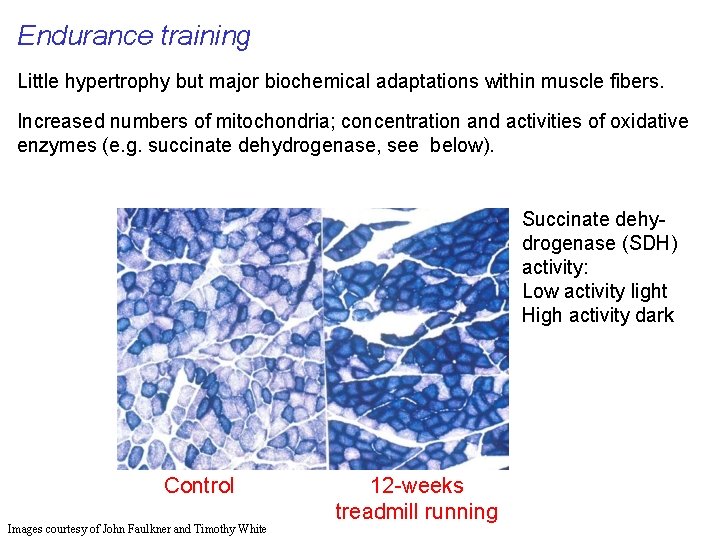
Endurance training Little hypertrophy but major biochemical adaptations within muscle fibers. Increased numbers of mitochondria; concentration and activities of oxidative enzymes (e. g. succinate dehydrogenase, see below). Succinate dehydrogenase (SDH) activity: Low activity light High activity dark Control Images courtesy of John Faulkner and Timothy White 12 -weeks treadmill running
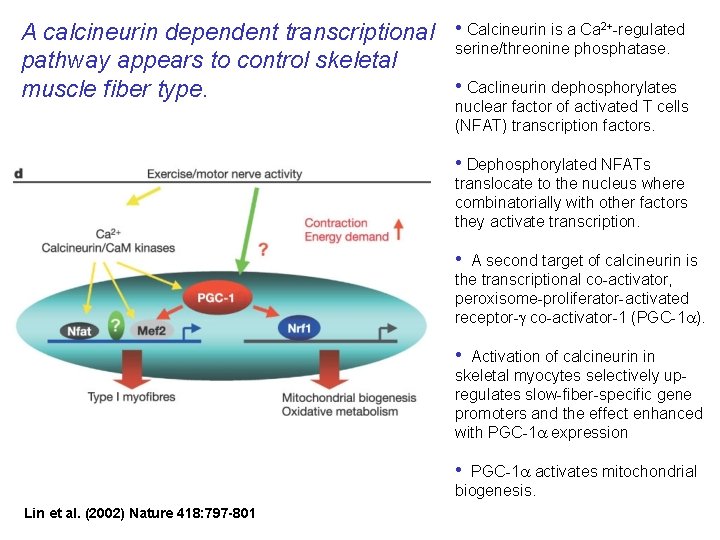
A calcineurin dependent transcriptional • Calcineurin is a Ca 2+-regulated serine/threonine phosphatase. pathway appears to control skeletal • Caclineurin dephosphorylates muscle fiber type. nuclear factor of activated T cells (NFAT) transcription factors. • Dephosphorylated NFATs translocate to the nucleus where combinatorially with other factors they activate transcription. • A second target of calcineurin is the transcriptional co-activator, peroxisome-proliferator-activated receptor-g co-activator-1 (PGC-1 a). • Activation of calcineurin in skeletal myocytes selectively upregulates slow-fiber-specific gene promoters and the effect enhanced with PGC-1 a expression • PGC-1 a activates mitochondrial biogenesis. Lin et al. (2002) Nature 418: 797 -801
A calcineurin dependent transcriptional pathway appears to control skeletal muscle fiber type. • Cyclosporin is widely used clinically to prevent rejection of transplanted tissues; patients develop skeletal muscle myopathy and loss of oxidative capacity. • Cyclosporin (and FK-506) are specific inhibitors of calcineurin and thereby block T cell activation. • Cyclosporin administration to intact animals promotes slowto-fast fiber transformation.

Increased use: strength training Early gains in strength appear to be predominantly due to neural factors…optimizing recruitment patterns. Long term gains almost solely the result of hypertrophy i. e. increased size.
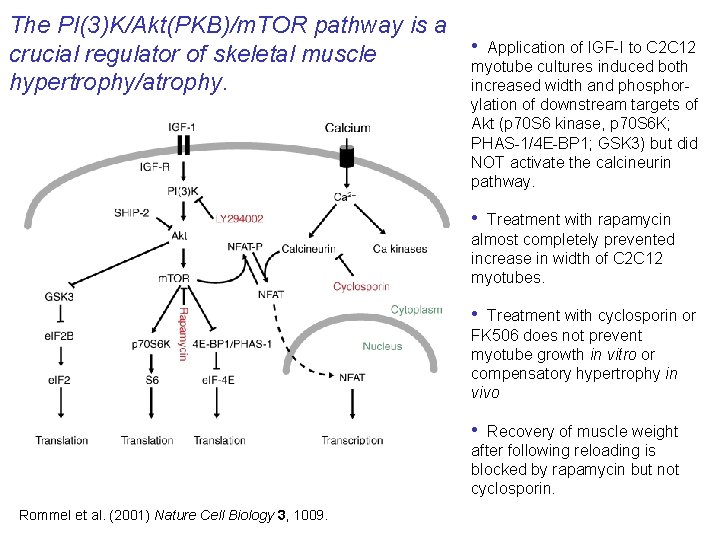
The PI(3)K/Akt(PKB)/m. TOR pathway is a crucial regulator of skeletal muscle hypertrophy/atrophy. • Application of IGF-I to C 2 C 12 myotube cultures induced both increased width and phosphorylation of downstream targets of Akt (p 70 S 6 kinase, p 70 S 6 K; PHAS-1/4 E-BP 1; GSK 3) but did NOT activate the calcineurin pathway. • Treatment with rapamycin almost completely prevented increase in width of C 2 C 12 myotubes. • Treatment with cyclosporin or FK 506 does not prevent myotube growth in vitro or compensatory hypertrophy in vivo • Recovery of muscle weight after following reloading is blocked by rapamycin but not cyclosporin. Rommel et al. (2001) Nature Cell Biology 3, 1009.
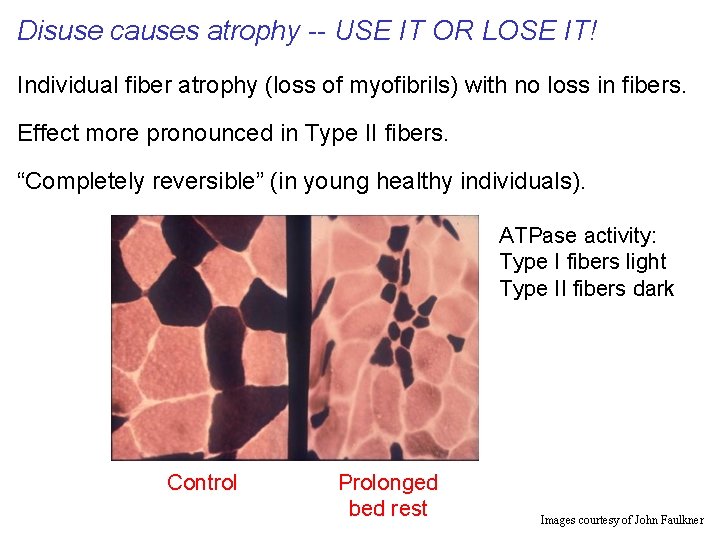
Disuse causes atrophy -- USE IT OR LOSE IT! Individual fiber atrophy (loss of myofibrils) with no loss in fibers. Effect more pronounced in Type II fibers. “Completely reversible” (in young healthy individuals). ATPase activity: Type I fibers light Type II fibers dark Control Prolonged bed rest Images courtesy of John Faulkner
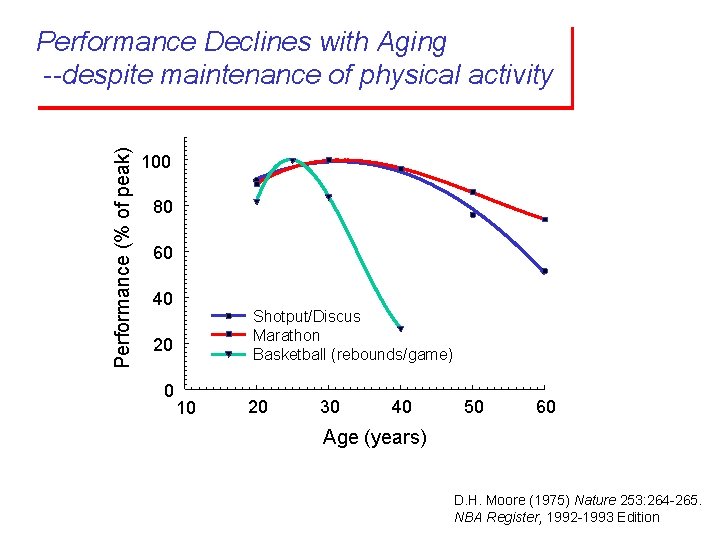
Performance (% of peak) Performance Declines with Aging --despite maintenance of physical activity 100 80 60 40 Shotput/Discus Marathon Basketball (rebounds/game) 20 0 10 20 30 40 50 60 Age (years) D. H. Moore (1975) Nature 253: 264 -265. NBA Register, 1992 -1993 Edition
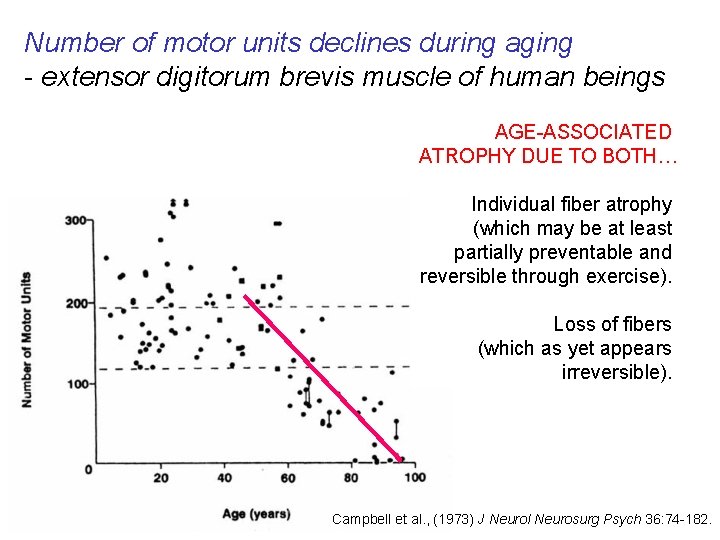
Number of motor units declines during aging - extensor digitorum brevis muscle of human beings AGE-ASSOCIATED ATROPHY DUE TO BOTH… Individual fiber atrophy (which may be at least partially preventable and reversible through exercise). Loss of fibers (which as yet appears irreversible). Campbell et al. , (1973) J Neurol Neurosurg Psych 36: 74 -182.
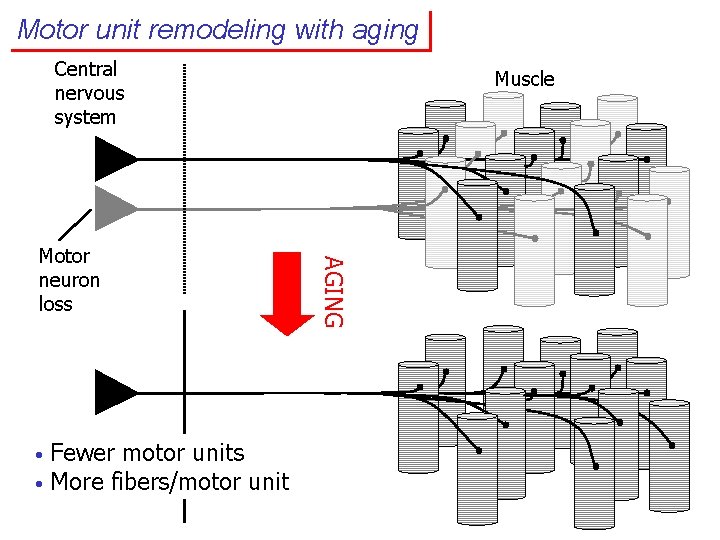
Motor unit remodeling with aging Central nervous system • • Fewer motor units More fibers/motor unit AGING Motor neuron loss Muscle
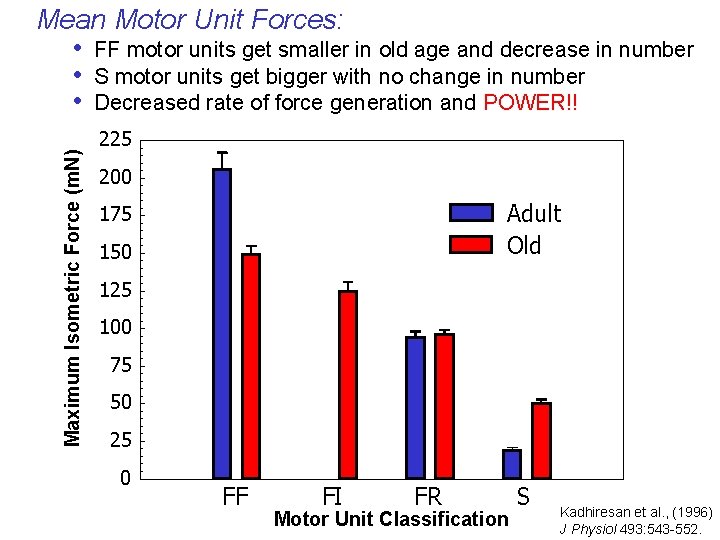
Maximum Isometric Force (m. N) Mean Motor Unit Forces: • FF motor units get smaller in old age and decrease in number • S motor units get bigger with no change in number • Decreased rate of force generation and POWER!! 225 200 Adult Old 175 150 125 100 75 50 25 0 FF FI FR Motor Unit Classification S Kadhiresan et al. , (1996) J Physiol 493: 543 -552.

Muscle injury may play a role in the development of atrophy with aging. • Muscles in old animals are more susceptible to contractioninduced injury than those in young or adult animals. • Muscles in old animals show delayed and impaired recovery following contraction-induced injury. • Following severe injury, muscles in old animals display prolonged, possibly irreversible, structural and functional deficits.
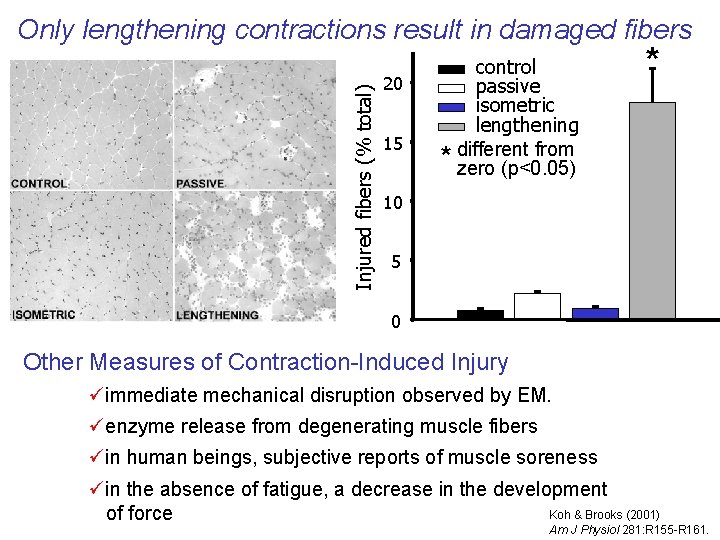
Injured fibers (% total) Only lengthening contractions result in damaged fibers 20 15 control passive isometric lengthening different from * zero (p<0. 05) * 10 5 0 Other Measures of Contraction-Induced Injury üimmediate mechanical disruption observed by EM. üenzyme release from degenerating muscle fibers üin human beings, subjective reports of muscle soreness üin the absence of fatigue, a decrease in the development Koh & Brooks (2001) of force Am J Physiol 281: R 155 -R 161.
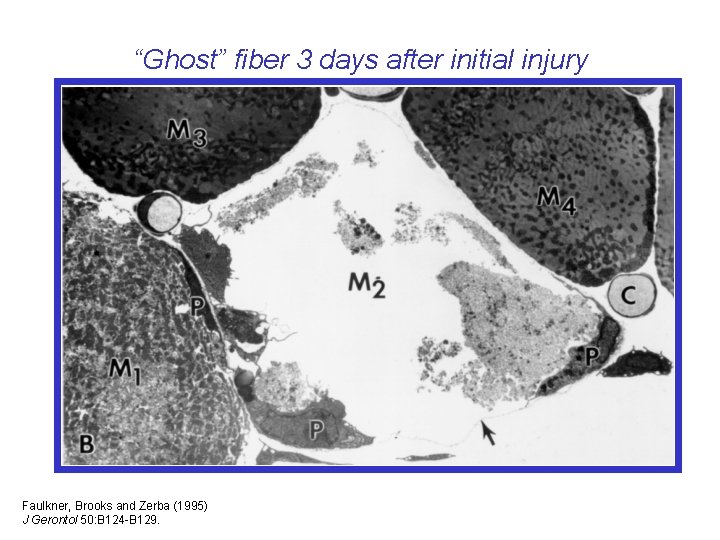
“Ghost” fiber 3 days after initial injury Faulkner, Brooks and Zerba (1995) J Gerontol 50: B 124 -B 129.
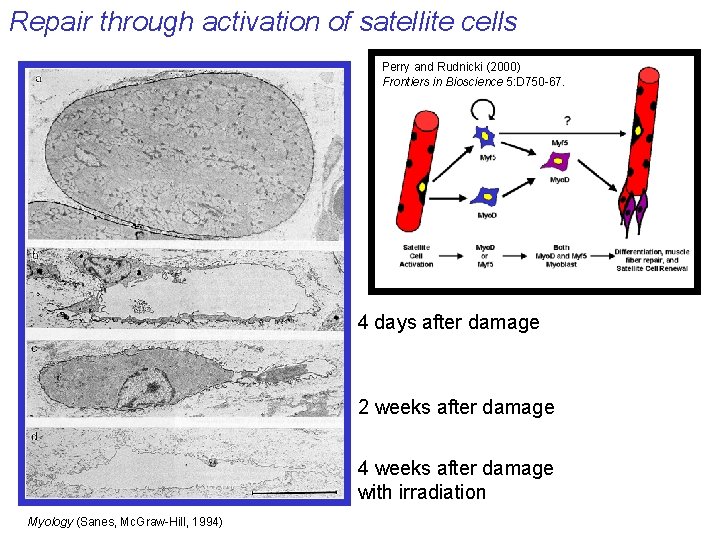
Repair through activation of satellite cells Perry and Rudnicki (2000) Frontiers in Bioscience 5: D 750 -67. 4 days after damage 2 weeks after damage 4 weeks after damage with irradiation Myology (Sanes, Mc. Graw-Hill, 1994)
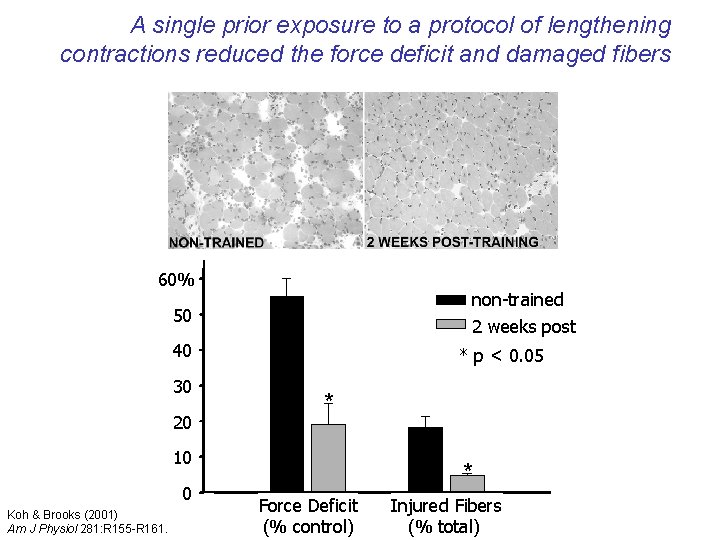
A single prior exposure to a protocol of lengthening contractions reduced the force deficit and damaged fibers 60% non-trained 2 weeks post 50 40 30 20 * p < 0. 05 * 10 0 Koh & Brooks (2001) Am J Physiol 281: R 155 -R 161. * Force Deficit (% control) Injured Fibers (% total)
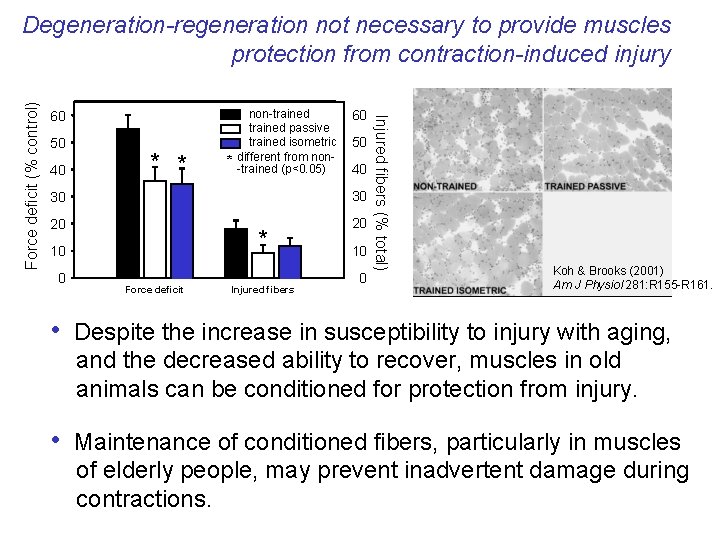
60 50 40 * * * non-trained passive trained isometric different from non-trained (p<0. 05) 60 50 40 30 30 20 20 * 10 0 Force deficit Injured fibers 10 0 Injured fibers (% total) Force deficit (% control) Degeneration-regeneration not necessary to provide muscles protection from contraction-induced injury Koh & Brooks (2001) Am J Physiol 281: R 155 -R 161. • Despite the increase in susceptibility to injury with aging, and the decreased ability to recover, muscles in old animals can be conditioned for protection from injury. • Maintenance of conditioned fibers, particularly in muscles of elderly people, may prevent inadvertent damage during contractions.
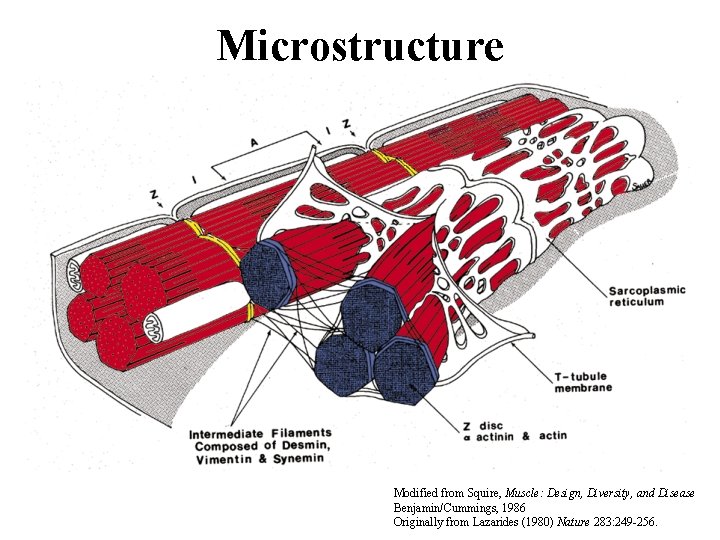
Microstructure Modified from Squire, Muscle: Design, Diversity, and Disease Benjamin/Cummings, 1986 Originally from Lazarides (1980) Nature 283: 249 -256.
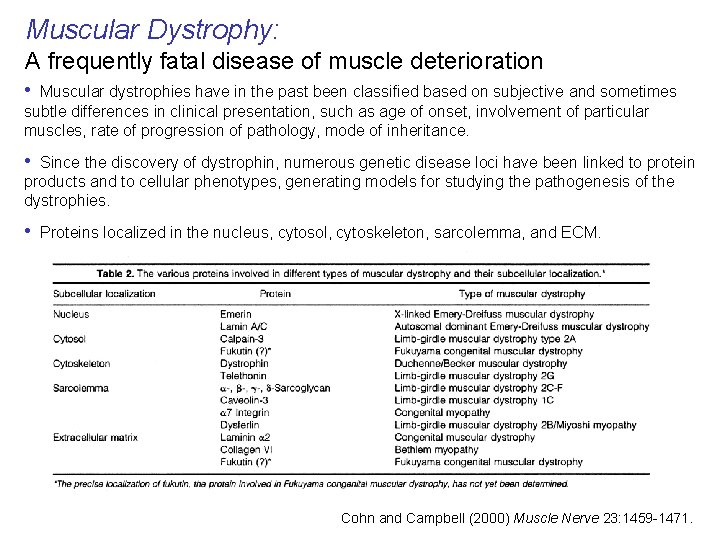
Muscular Dystrophy: A frequently fatal disease of muscle deterioration • Muscular dystrophies have in the past been classified based on subjective and sometimes subtle differences in clinical presentation, such as age of onset, involvement of particular muscles, rate of progression of pathology, mode of inheritance. • Since the discovery of dystrophin, numerous genetic disease loci have been linked to protein products and to cellular phenotypes, generating models for studying the pathogenesis of the dystrophies. • Proteins localized in the nucleus, cytosol, cytoskeleton, sarcolemma, and ECM. Cohn and Campbell (2000) Muscle Nerve 23: 1459 -1471.
Dystrophin function: transmission of force to extracellular matrix DGC dystrophin dystroglycan (a and b) sarcoglycans (a, b, g, d) syntrophins (a, b 1) dystrobrevins (a, b) sarcospan laminin-a 2 (merosin) (Some components of the dystrophin glycoprotein complex are relatively recent discoveries, so one cannot assume that all players are yet known. ) Cohn and Campbell (2000) Muscle Nerve 23: 1459 -1471.
- Slides: 32