Sizing and planning in EVAR Endograft selection when




















- Slides: 32

Sizing and planning in EVAR Endograft selection when planning in s. EVAR: Endografts’ characteristics and limitations Konstantinos G. Moulakakis MD, Ph. D, FEBVS Consultant, Vascular Surgeon Department of Vascular Surgery, Medical School, University of Athens

Introduction § (EVAR) technology has undergone intense scrutiny, which has allowed the development and refinement of many generations of endografts LAST GENERATION DEVICES § Increased deliverability § Lower profile § Ease of use § Durability ?

Standard EVAR 20% to 69% of patients have been reported to meet the criteria.

Endograft selection when planning in s. EVAR 1. Comparison of IFU 2. Suitability in certain anatomical aspects 3. Endografts’ characteristics and limitations

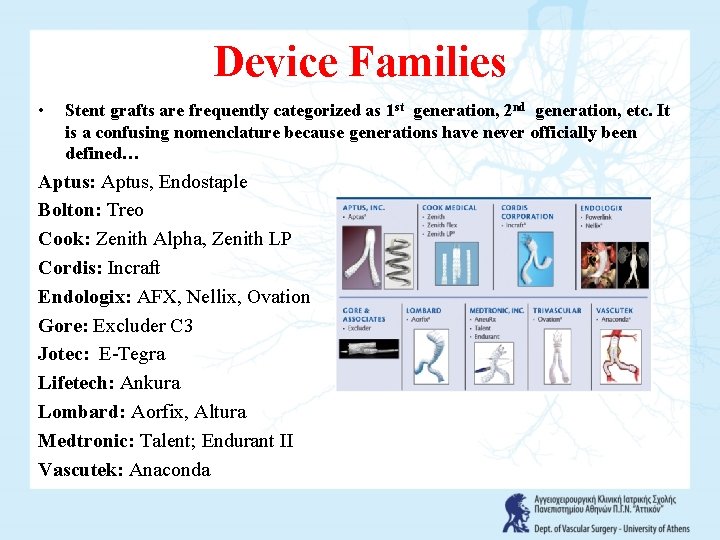
Device Families • Stent grafts are frequently categorized as 1 st generation, 2 nd generation, etc. It is a confusing nomenclature because generations have never officially been defined… Aptus: Aptus, Endostaple Bolton: Treo Cook: Zenith Alpha, Zenith LP Cordis: Incraft Endologix: AFX, Nellix, Ovation Gore: Excluder C 3 Jotec: E-Tegra Lifetech: Ankura Lombard: Aorfix, Altura Medtronic: Talent; Endurant II Vascutek: Anaconda

1. Analysis-Comparison of last generation devices inside the IFU (1) Stent Graft Endurant II/IIS Endurant Zenith Excluder II/Iis + Heli. FX Alpha Flex ≥ 4 mm , <10 mm ≥ 15 mm Treo AFX 2 ≥ 10 mm ≥ 15 mm ≤ 60, ≤ 75 ≤ 60 Neck length ≥ 10 mm IR angulation IR neck diameter ≤ 60, ≤ 75 ≤ 60 19 -32 mm 18 -32 mm 19 -32 mm 17 -32 mm 18 -32 mm 8 -25 mm 8 -20 mm 10 -23 mm ≥ 15 mm ≥ 10 mm ≥ 15 mm Iliac diameter Iliac length for sealing ≤ 60 ≥ 10 mm

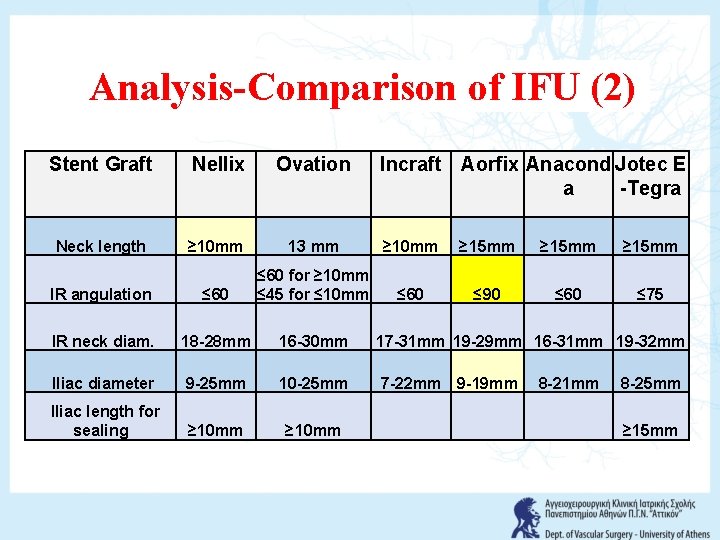
Analysis-Comparison of IFU (2) Stent Graft Nellix Ovation Incraft Aorfix Anacond Jotec E a -Tegra Neck length ≥ 10 mm 13 mm ≥ 10 mm ≥ 15 mm IR angulation ≤ 60 for ≥ 10 mm ≤ 45 for ≤ 10 mm ≤ 60 ≤ 90 ≤ 60 ≤ 75 IR neck diam. 18 -28 mm 16 -30 mm 17 -31 mm 19 -29 mm 16 -31 mm 19 -32 mm Iliac diameter 9 -25 mm 10 -25 mm 7 -22 mm 9 -19 mm 8 -21 mm 8 -25 mm Iliac length for sealing ≥ 10 mm ≥ 15 mm

So…regarding the Length of the Neck • NECK LENGTH: The majority of endografts need > 15 mm of neck length Endurant, Treo and Incraft can be implanted in 10 mm - 15 mm The Endurant has CE Mark for 4 -10 mm in combination with stapler • NECK DIAMETER: The majority of stent-grafts can treat 18 -32 mm diameter of the neck ( Nellix and Aortfix, up to 28 -29 mm)

Infrarenal or Suprarenal fixation? In which anatomy?

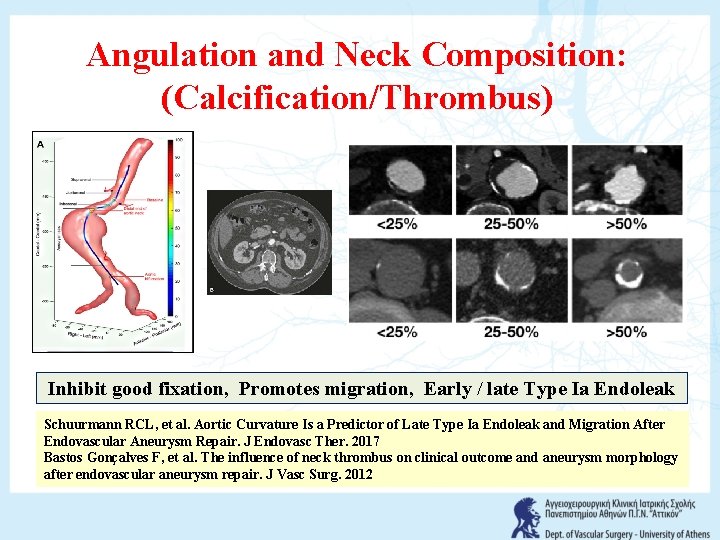
Angulation and Neck Composition: (Calcification/Thrombus) Inhibit good fixation, Promotes migration, Early / late Type Ia Endoleak Schuurmann RCL, et al. Aortic Curvature Is a Predictor of Late Type Ia Endoleak and Migration After Endovascular Aneurysm Repair. J Endovasc Ther. 2017 Bastos Gonçalves F, et al. The influence of neck thrombus on clinical outcome and aneurysm morphology after endovascular aneurysm repair. J Vasc Surg. 2012

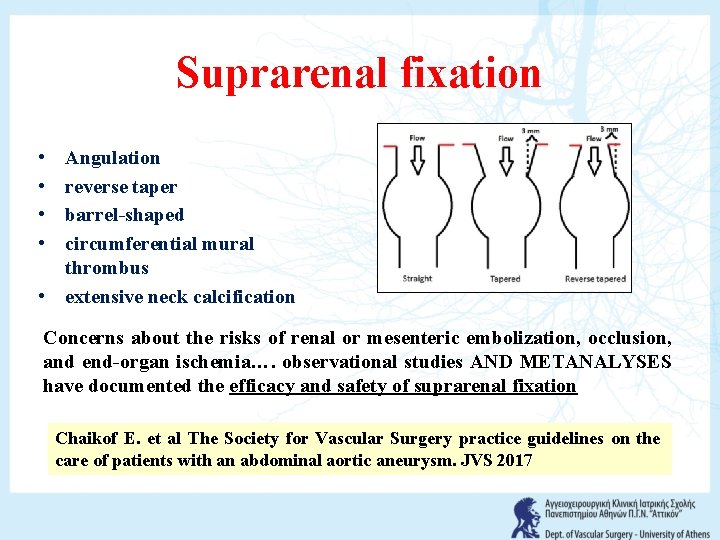
Suprarenal fixation • • Angulation reverse taper barrel-shaped circumferential mural thrombus • extensive neck calcification Concerns about the risks of renal or mesenteric embolization, occlusion, and end-organ ischemia…. observational studies AND METANALYSES have documented the efficacy and safety of suprarenal fixation Chaikof E. et al The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. JVS 2017

Angulation 60 -75° M-Shape configuration of the skeleton of the stent -graft Require a neck length > 15 mm Endurant II, Medtronic Treo, Bolton

Angulation 90° • The Aorfix device incorporating the “fish mouth” design. • Concentrated concentric rings within the first 8 mm of the device, as well as four aggressive sets of hooks that provide proximal fixation • Designed and tested to treat highly angulated aortic necks • Anaconda Terumo has received CE Mark Aortfix PYTHAGORAS U. S. Clinical Trial

Access-related issues • Access-related complications, 5% to 17%. • Common exclusion criterion for EVAR and the leading cause of conversion to open repair. • Small, narrow, calcified, have a higher rate of limb occlusion, limb stenosis, and limb kinking. • Severe Tortuosity • EUROSTAR experience, 28. 6% of the conversions to open repair occurred because of injury during the introduction of the device. 1. Murray D, Ghosh J, Khwaja N, et al. Access for endovascular aneurysm repair. J Endovasc Ther. 2006 2. Cuypers PW, et al. Which factors increase the risk of conversion to open surgery following endovascular abdominal aortic aneurysm repair? The EUROSTAR collaborators. Eur J Vasc Endovasc Surg. 2000

In small, narrow, calcified iliac arteries 14 -16 Fr have become available Ovation Incraft

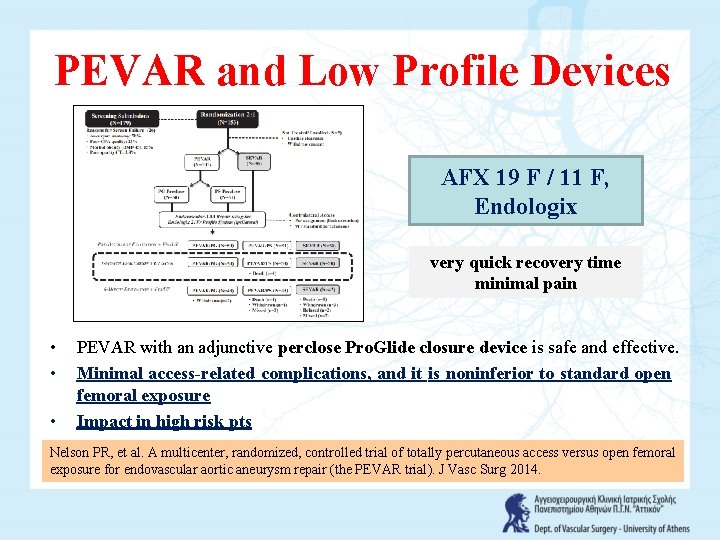
PEVAR and Low Profile Devices AFX 19 F / 11 F, Endologix very quick recovery time minimal pain • • • PEVAR with an adjunctive perclose Pro. Glide closure device is safe and effective. Minimal access-related complications, and it is noninferior to standard open femoral exposure Impact in high risk pts Nelson PR, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg 2014.

Tortuous iliac arteries • The architectural design of the supporting framework has an impact on limb patency (helical shape stent design) • The conformability of PTFE should allow the material to collapse or “accordion” very easily, whereas the rigidity of PET would likely make it more prone to kinking and flow disturbances. Ovation C 3 Excluder Advanced with sheath Aortfix

Tortuous iliac arteries • Helical limb structure maintains patency in tortuous anatomies • The circular design delivers unmatched flexibility while maintaining its luminal size even in extreme angulations

When Contralateral cannulation might be difficult…in large AAAs with tortuous iliac arteries… Anaconda, Terumo C 3 Excluder Repositionable, Magnetic wire facilitating the contralateral cannulation

Nellix system CONCEPT aims to treat the sac • • Aortic aneurysm with a blood lumen diameter of ≤ 70 mm Ratio of maximum aortic aneurysm diameter to maximum aortic blood lumen diameter ≤ 1. 4 • Does not rely on proximal-neck fixation and seal • Designed to mitigate endoleak of any type • May prevent acute sac thrombosis Promising concept, long term data will be soon available

Problems still not fully addressed with current s. EVAR devices • Neck limitations still represent a concern and have not changed even with the last generation devices. • Most grafts are now made of nitinol stents instead of stainless steel and the price we pay is somewhat lower visibility, lower radial force. • Reduction in access size is based on changes in fabric and stent structure/composition, which can lead to issues with durability.

Conclusions • Meticulous planning according to the IFU is an important part of planning for standard EVAR attempting a favorable outcome • Suprarenal fixation is indicated in necks with angulation, reverse taper or barrel-shaped configuration, circumferential mural thrombus, and extensive neck calcification • In small, narrow, calcified iliac arteries low profile devices are indicated. • In tortuous iliac arteries PTFE and circular design limbs have better results compared to Woven and non circular design limbs • Vascular surgeons must use devices with which are sufficiently familiarized and have an experience with good results

Thank you for your attention


Device Families • Stent grafts are frequently categorized as first generation, second generation, etc. It is a confusing nomenclature because generations have never officially been defined. It may prove more useful to describe devices according to their origin, or families. EVAR device families can therefore be defined by their manufacturers (Figure 5): • Aptus: Endostaple, FDA-approved but not the graft; • Bolton: Treovance, not approved; • Cook: Zenith; Zenith Flex, FDA-approved; Zenith LP, not approved; and now Zenith Fenestrated, FDA-approved - customized; • Cordis: Incraft, not approved; • Endologix: Powerlink, FDA-approved; Nellix, not approved; • Gore: Excluder; Excluder C 3, FDA-approved; • Lombard: Aorfix, not approved; • Medtronic: Aneu. Rx; Talent; Endurant, all FDA-approved;

Infrarenal fixation • Most endografts that are dependent on infrarenal fixation require a proximal sealing zone of at least 15 mm in length, a neck diameter <32 mm, and a neck angulation of <60 degrees. • Several devices now report efficacy with shorter neck lengths and more severe levels of angulations.


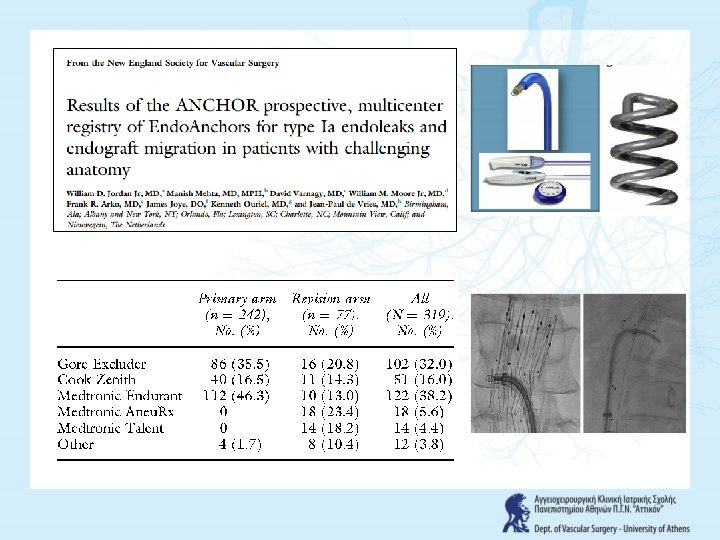
FREEDOM FROM TYPE Ia ENDOLEAK 95% 77% Jordan et al. Vascular 2016; 24: 177 -86

Narrow and heavily calcified iliacs ? Tourtous Iliacs?

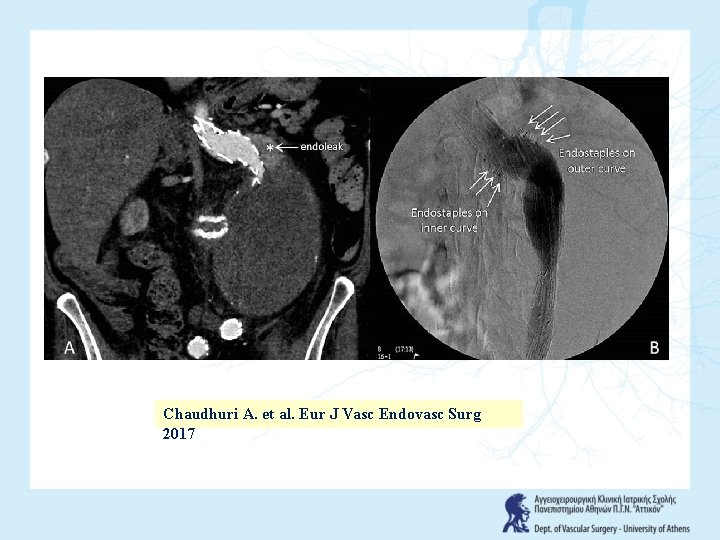
Chaudhuri A. et al. Eur J Vasc Endovasc Surg 2017

• 1. What has been addressed with current devises? • What has not been addressed? • Limitations and concerns for current endografts
