Size Exclusion Chromatography SEC Gelfiltration chromatography Size Exclusion






- Slides: 20

Size Exclusion Chromatography (SEC) Gel-filtration chromatography.

Size Exclusion Chrom. • Molecules are separated according to differences in their size as they pass through the gelfiltration matrix • Non-adsorption technique • Ideally no interaction between matrix and molecules • Polymer beads composed of cross-linked dextran (dextrose) which is highly porous

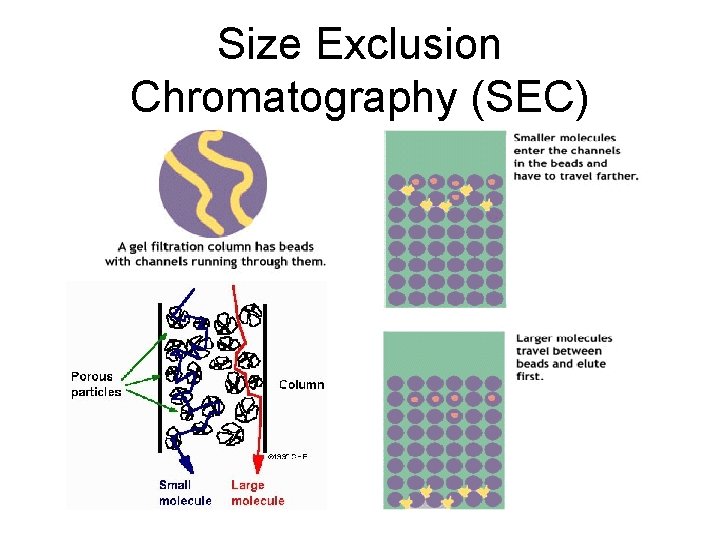
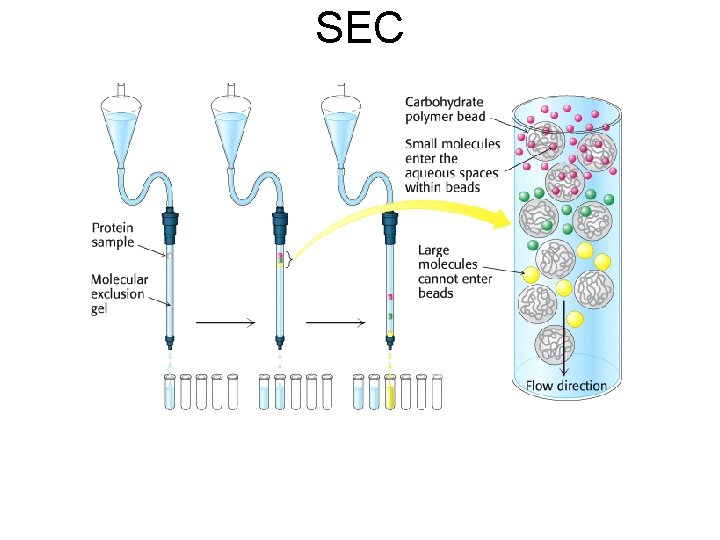
SEC • Molecules with diameter greater than the largest pores within resin material are unable to enter the particle. Therefore they pass through the smallest accessible volume, they travel through the column from top to bottom fastest and elute first • Smaller molecules enter pores and access pores within the resin particles. Pass through the larger accessible volume within the column and beads. Therefore elute later. • Elution is in order of decreasing molecular weight.

Size Exclusion Chromatography (SEC)

SEC

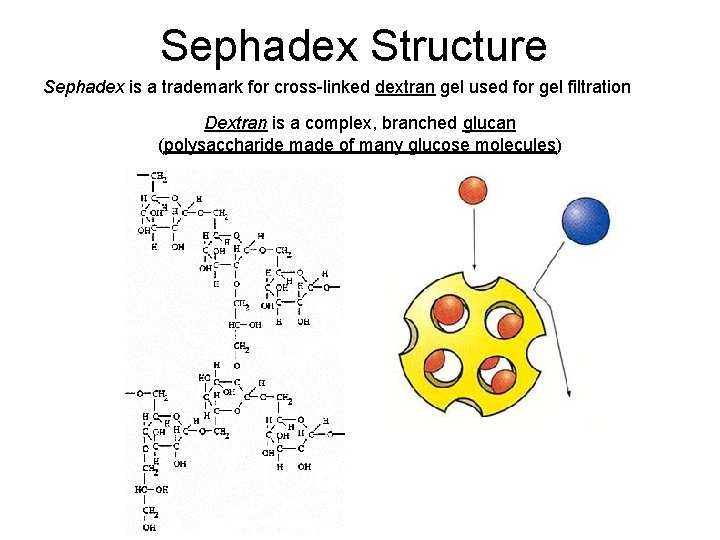
Sephadex Structure Sephadex is a trademark for cross-linked dextran gel used for gel filtration Dextran is a complex, branched glucan (polysaccharide made of many glucose molecules)

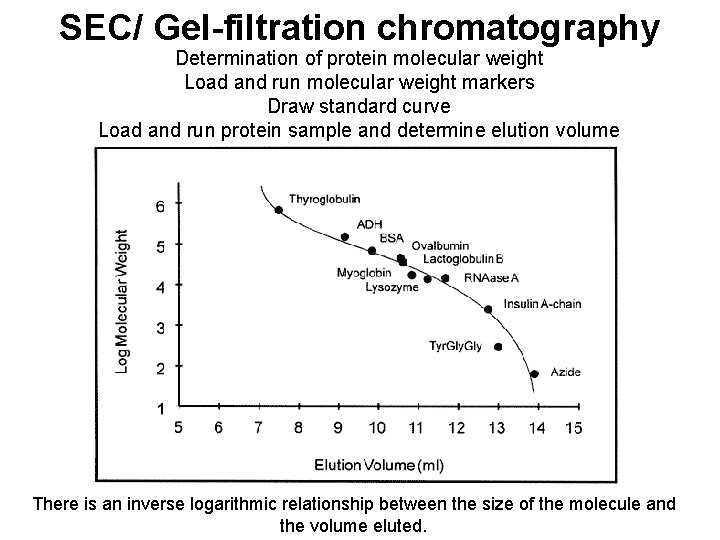
SEC/ Gel-filtration chromatography Determination of protein molecular weight Load and run molecular weight markers Draw standard curve Load and run protein sample and determine elution volume There is an inverse logarithmic relationship between the size of the molecule and the volume eluted.

Application of SEC • Protein separation and purification • Molecular weight determination • Determination of Oligomeric state (dimer/trimer etc) • Protein-Protein interaction

Excercise • 10, 20, 40, 50 k. Da protein • Which will elute first in gel filtration? • Which will elute last in gel filtration?

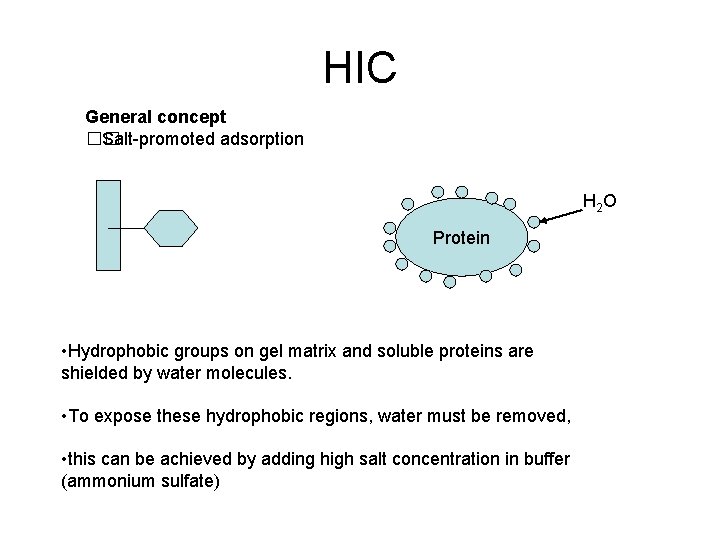
HIC (Hydrophobic interaction chromatography) Principle • Separation of substances is based on their hydrophobic interaction with hydrophobic groups attached to an uncharged gel matrix • Hydrophobic groups on proteins are sufficiently exposed to bind to the hydrophobic groups on the matrix. • How is this achieved?

HIC General concept �� Salt-promoted adsorption H 2 O Protein • Hydrophobic groups on gel matrix and soluble proteins are shielded by water molecules. • To expose these hydrophobic regions, water must be removed, • this can be achieved by adding high salt concentration in buffer (ammonium sulfate)

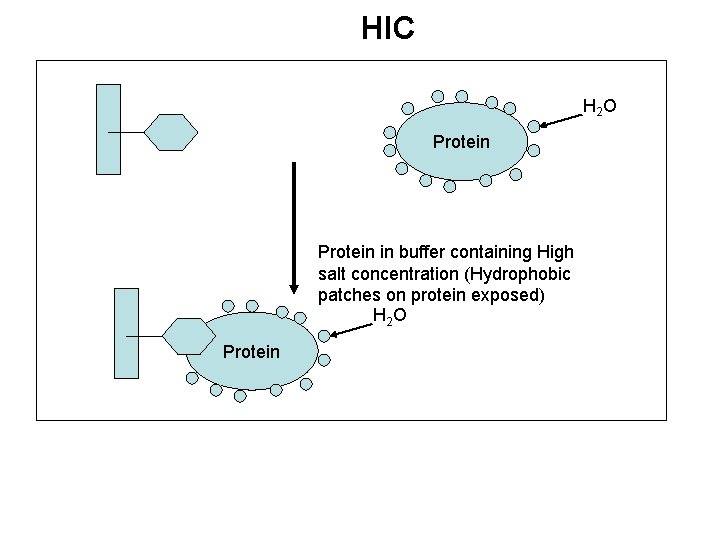
HIC H 2 O Protein in buffer containing High salt concentration (Hydrophobic patches on protein exposed) H 2 O Protein

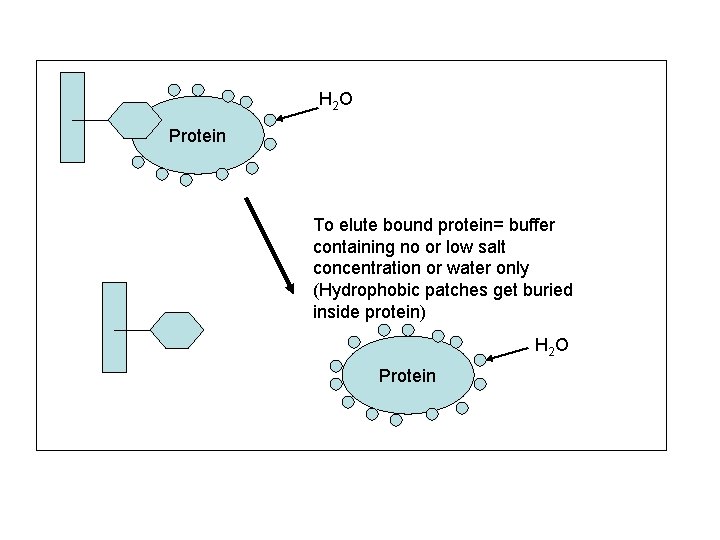
H 2 O Protein To elute bound protein= buffer containing no or low salt concentration or water only (Hydrophobic patches get buried inside protein) H 2 O Protein

HIC Similar to ion-exchange chromatography except that column material contains aromatic or aliphatic alkyl groups (phenyl, Butyl, Octyl) Protein attach in the presence of high salt concentration (1 M ammonium sulfate/ 2 M Na. Cl) Bound proteins elute using buffer containing low salt concentration or no salt

Salting out is a method of separating proteins based on the principle that proteins are less soluble at high salt concentrations. The salt concentration needed for the protein to precipitate out of the solution differs from protein to protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed.

Principle of salting out • There are hydrophobic amino acids and hydrophilic amino acids in protein molecules. • After protein folding in aqueous solution, hydrophobic amino acids usually form protected hydrophobic areas while hydrophilic amino acids interact with the molecules of solvation and allow proteins to form hydrogen bonds with the surrounding water molecules. • If enough of the protein surface is hydrophilic, the protein can be dissolved in water. • When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the charged part of the protein. • As a result of the increased demand for solvent molecules, the protein-protein interactions are stronger than the solvent-solute interactions; the protein molecules coagulate by forming hydrophobic interactions with each other. This process is known as salting out. • As different proteins have different compositions of amino acids, different protein molecules precipitate at different concentrations of salt solution. Ammonium sulfate precipitation

What follows salting out step? • Need to remove salt • Following a salting-out step, the solution will contain a high concentration of salt that can be disruptive to subsequent chromatographic steps. • Dialysis

Dialysis Movement of molecules by diffusion from high concentration to low concentration through a semi-permeable membrane. • Only those molecules that are small enough to fit through the membrane pores are able move through the membrane and reach equilibrium with the entire volume of solution in the system • By contrast, large molecules that cannot pass through the membrane pores will remain on the same side of the membrane as they were when dialysis was initiated. • To remove additional unwanted substance, it is necessary to replace the dialysis buffer so that a new concentration gradient can be established. • Once the buffer is changed, movement of particles from high (inside the membrane) to low (outside the membrane) concentration will resume until equilibrium is once again reached.

Separation based on SIZE Dialysis: • for separating proteins from small molecules & ions Small molecules (blue) (buffer ions, small organic molecules, salt etc. ) pass through membrane, diffusing into surrounding medium. • Dialysate (buffer in which dialysis bag is suspended) can be changed frequently --> serial dilution, getting rid of unwanted small molecules and ions. • Dialysis membranes with different pore sizes available

Factors Affecting Dialysis Rate Factors that affect the completeness of dialysis include (1) dialysis buffer volume, (2) buffer composition, (3) the number of buffer changes, (4) time, (5) temperature and (6) particle size vs. membrane pore size.