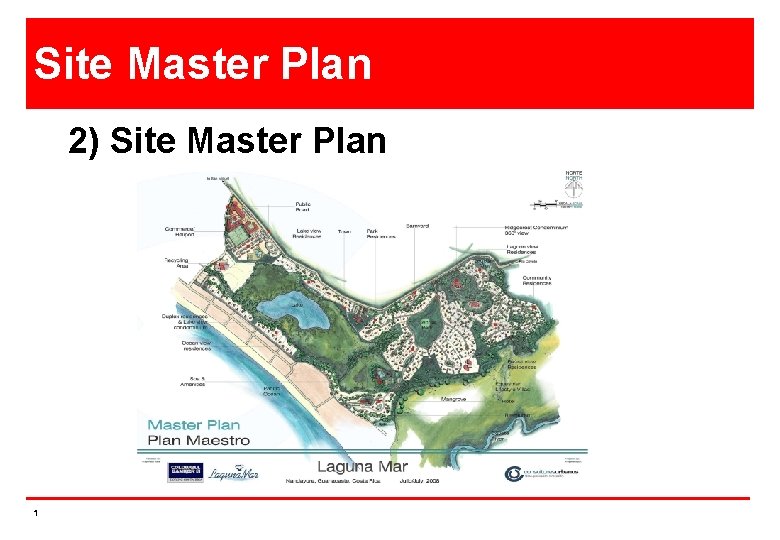
Site Master Plan 2 Site Master Plan 1
























- Slides: 55

Site Master Plan 2) Site Master Plan 1

Site Master Planning Rationale n n 2 Serious investment – long project duration – good long term planning essential Planning, and even investment frequently required before product goes into phase 3 Combination of specific and generic elements – flexibility essential Assume full scope site including fill/ finish even though later is frequently separate

Site Master Planning Assumptions n n 3 Product and Process ranges have been agreed We have completed a build/ buy/ outsource analysis and have noted current world capacity position Focus on Monoclonals and our process will follow broad Mab guidelines Our broad philosophies are established in terms of: GMP, Single v’s Multi-product, Expandability etc

Site Master Planning Prerequisites n n n n 4 Defined product characteristics Broad product demand parameters e. g. dose, patients population etc Our market potential and penetration Broad process parameters Location philosophy Scope of investment on our site Site selection is nor a prerequisite for master planning

Site Master Planning Site Scope n n 5 Filling process and process utilities are core, usually in single building Site usually includes process development, QA, QC, warehouse, utilities, personnel facilities and technical management are needed Fill finish (stages from bulk to dose form) are optional as are packaging and distribution R&D, high level admin, finance, sales, etc are optional. Decision between ‘flagship facility’ and ‘lean, mean’ functional unit’ is essential early

Site Master Planning Flexibility, Expandability, Future Proofing n n n 6 Key decisions are needed here – must be a cap! Define how much to install, how much to provide space for, how much to service for? Philosophy on expandability, modularity, flexibility is needed Define modularity – can range from slotting in a serviced vessel to stand alone modular segments These decisions are fundamental, they can determine the success or failure of the facility and must be in the context of 5 years in the future

Site Master Planning Determine Site Requirements n n n 7 Sizing is based on output requirements. These derive from dose x patient population and this applies to all facility levels (pilot, chemical, full scale) Dose may be in vials, pre-filled syringes or other forms. Main form is lyophilised product in vials. Separate logistics apply to sizing fill/ finish facility including formulation, bulk preparation, filling, lyophilization

Site Master Planning Sizing & Shaping n n n 8 SMP is a collection of ‘boxes’ of varying size and shape arranged in a functional, orderly and aseptically pleasing configuration Sizing individual boxes requires analysis of the process requirements, sizing and arranging the equipment and services and configuring the internal workings for optimal personnel and material movement Arranging the boxes needs an analysis of inter box movements and requirements for adjacency, closeness etc. Expansion philosophy is applied here

Site Master Planning Broad Rules n n n 9 Minimise process transfers – address CIP considerations Recognise basic contiguity needs e. g. airlocks, gowning areas, autoclaves, sterilizers vs protected core All material movements should be under cover (link corridors) HVAC plant rooms should be above classified areas – minimise duct runs Buffer Prep and clean utilities to be adjacent to process areas Design from process core outwards and from output requirements backwards

Site Master Planning Configuration Philosophy n n n n 10 Various layers are placed around production core – based on proximity requirements Layers Adjoining Adjacent in same building Adjacent – linked by corridor Reasonably close – preferably covered access Remote

Site Master Planning Layer 1 – Contiguous/ Adjoining n n n n 11 Airlocks for materials/ components Secondary change areas Autoclaves and sterilisers CIP skids Process development/ pilot plant Air handlers and distribution Process drains

Site Master Planning Layer 2 – Same Building n n n 12 Clean utilities Buffer Preparation QA In-Process Lab Some personnel facilities on large site

Site Master Planning Layer 3 – Adjacent n n n 13 Warehouse – separate linked building Cold Store – Central Primary change for operators Maintenance workshop Cafeteria

Site Master Planning Layer 4 - Near - Preferably Linked n n n 14 QC labs Engineering HR Administration Security EHS

Site Master Planning Layer 5 – Remote or Distributed n n n 15 n Utilities - general - steam, comp air, potable water, chilling, fire water Effluent treating Fire water storage and pumping Tank farms Car parks / roads Gas storage

Site Master Planning Configuration Options n n 16 Single Building – most economical but not good option for large scale – limits expansion and mixes incompatible operations Spin link corridor – probably ideal – buildings attached on 2 sides – can extend length and travel distances if expansion space is required Rectangular loop link – also good – again can limit expansion but reduces some travel distances Radial – good concept but impractical for implementation

Site Master Planning Buffer Make Up n n 17 Separate areas for filling and buffer make up Requires solids handling capability Generally all buffer solutions are presented aseptically to Formulation/ Filling Area adjacent to Formulation/ Filling

Site Master Planning Other Considerations n n n 18 Chilled Vessels for storage Intermediate cold storage Possible separate air handling systems Separate entry and exit facilities for materials and personnel Decontamination of exiting clothing and equipment Later stages require aseptic handing and freezing facilities

Site Master Planning Sizing Buffer Make Up n n n 19 Requires prep and hold vessels Material handling and dust control Transfer by aseptic filtration

Site Master Planning Sizing Process Utilities n n n 20 WFI requirements calculated on flows demands and minimum circulation rates Storage vessels can be 10 -20 m 3 – required balanced calculation between storage and generation CIP demands determine capacities – high instant flow rates Clean Steam based on max SIP load – usually empty vessel – made from clean steam Clean gases based on process demands and some transfers – all filtered

Site Master Planning General Utilities n n n 21 Normally located centrally – CUB concept Allow for pipe-racks (usually in link corridors) Includes steam, chilling, cooling, compressed air, power, effluent treatment

Site Master Planning 22 Utilities Sizing – Ranges n Steam 40 -60 tons/ hr @ 0 bar n Chilling 20 -30 MW n Cooling 40 -60 MW n Compressed Air 4, 000 -6, 000 Nm 3 / hr n Divide all into modules – min 3 equal sized units n Power 20 -30 MVA – Supply at H. T.

Site Master Planning Building Sizes - Ranges n n Sizing based on previous slides – boxes must be sized before being arranged Multi-floor arrangements more economical within reason In all cases, top floor for plant with prospect of additional plant space on 1 side Crude sizes for 100, 000 L facility approx, 60, 000 – 80, 000 m 2 Ø Ø Ø 23 Bulk Production/ DSP 20, 000 m 2 (3 -4 floors) Fill/ Finish 10, 000 – 15, 000 m 2 (3 floors) Warehouse 10, 000 m 2 (1 floor @ 12 m) CUB 5, 000 – 10, 000 m 2 (1 floor) Development 9, 000 m 2 (3 floors) Admin 5, 000 – 10, 000 m 2 (3 floors)

Site Master Planning Expansion n n 24 Clear policy needed – otherwise high ‘fuzz’ potential Built space essential to avoid future disruption Adjacent space needed for all functions Constructability of expansion needs some study even at GMP: stage

Technology Transfer 3) Technology Transfer 25

Overview § § § 26 Introduction Critical GMP Drivers Tech Transfer Process Team Participation Tech Transfer Completion & Success

Technology Transfer Definition Transfer of all necessary information and support to successfully manufacture and evaluate the transferred product, process or analytical test method, at the selected manufacturing site(s). 27 A successful transfer is a collaborative effort among cross-functional technology teams representing various site disciplines with communication as a cornerstone to that success.

What is Technology Transfer ? n Product Technology Transfer Business Considerations n Shipping Considerations n Material Availability n Regulatory Considerations n Strategic Facility considerations n n Process/ Method Technology Transfer Specific Tests or Process Steps n Specifications n Process/ method development & validation n Process related Facility Considerations n 28

What is Technology Transfer ? Why conduct Technology Transfer? § Systematic approach § Define responsibilities § Ensure process/ method validation § c. GMP Drivers Business Considerations ? § Speed to Market § Lower Cost § Improved Customer Service § Compliance Obligations 29

Critical GMP Drivers n No Specific Reference in 21 CFR 210/211 30

Critical GMP Drivers n Indirect References in 21 CFR 211. 160 Subpart I, Laboratory Controls, General Requirements n 211. 186 Subpart J, Records & Reports, Master Production and Control Reports n 211. 110 Subpart F, Production and Process Controls, Sampling and Testing of inprocess materials and drug products. n 211. 100 Subpart F, Production and Process Controls, Written Procedures, Deviations n 31

Critical GMP Drivers n Eudralex Vol 4 Chapter 7 – Contract Manufacture and Analysis n 32 7. 10 “A contract. . . specifies their respective responsibilities relating to manufacture and control of the product. Technical aspects of the contract should be drawn up by persons suitably knowledgeable in pharmaceutical technology, analysis and Good Manufacturing Practice”

Critical GMP Drivers Capture Critical GMP Drivers under n Training Subject Matter Experts (SMEs) n Understand Science & Technology n n Documentation n Technical Documentation Package n Document Robustness n Document consistency of control n 33 Platform for Validation

Establishing a TT Process Who conducts Technology Transfer ? § Originating – Receiving sites § R&D / Commercial § Intra Company transfer ØSite – Site or Internal § Transfer to External Business Partners 34

Technology Transfer Roadmap How is Technology Transfer Conducted? n Governance n n TT Plan n n Strategic Overview Experimental Outline and Validation Approach Accountability Table Technical Documentation Package n n 35 Technology Development & Transfer (TD&T) TT Report(s) Technical info & data generated in support of strategic plan

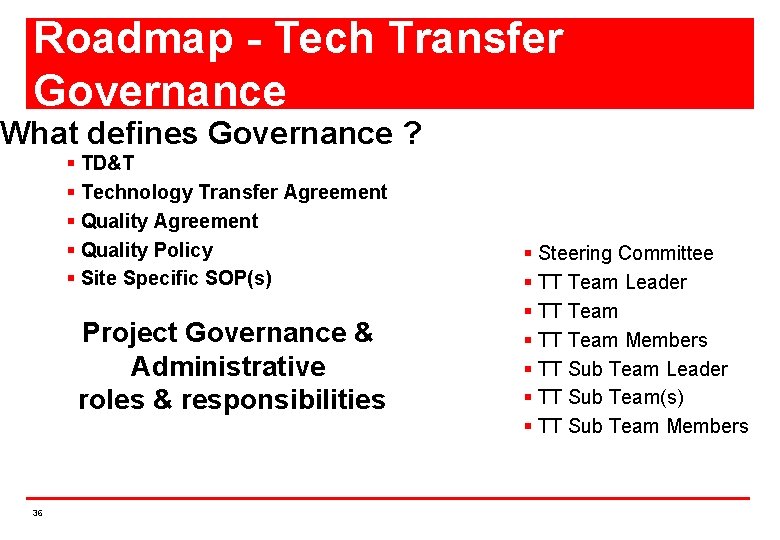
Roadmap - Tech Transfer Governance What defines Governance ? § TD&T § Technology Transfer Agreement § Quality Policy § Site Specific SOP(s) Project Governance & Administrative roles & responsibilities 36 § Steering Committee § TT Team Leader § TT Team Members § TT Sub Team Leader § TT Sub Team(s) § TT Sub Team Members

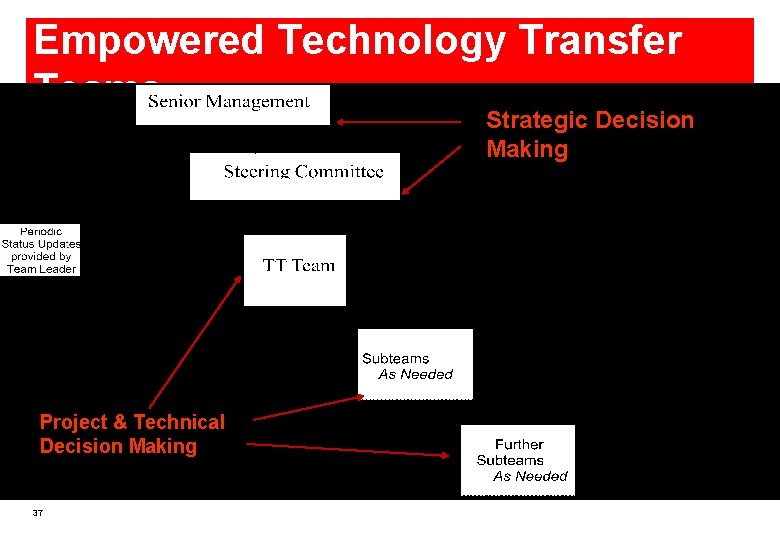
Empowered Technology Transfer Teams Strategic Decision Making Project & Technical Decision Making 37

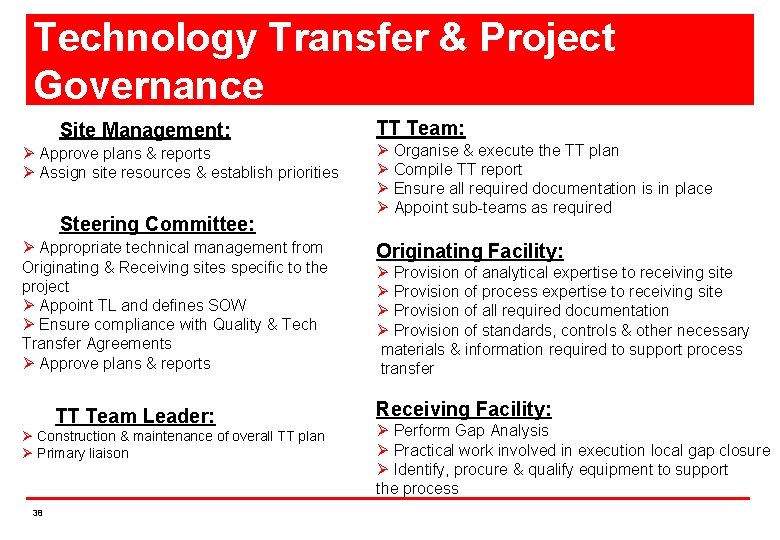
Technology Transfer & Project Governance Site Management: Ø Approve plans & reports Ø Assign site resources & establish priorities Steering Committee: Ø Appropriate technical management from Originating & Receiving sites specific to the project Ø Appoint TL and defines SOW Ø Ensure compliance with Quality & Tech Transfer Agreements Ø Approve plans & reports TT Team Leader: Ø Construction & maintenance of overall TT plan Ø Primary liaison 38 TT Team: Ø Organise & execute the TT plan Ø Compile TT report Ø Ensure all required documentation is in place Ø Appoint sub-teams as required Originating Facility: Ø Provision of analytical expertise to receiving site Ø Provision of process expertise to receiving site Ø Provision of all required documentation Ø Provision of standards, controls & other necessary materials & information required to support process transfer Receiving Facility: Ø Perform Gap Analysis Ø Practical work involved in execution local gap closure Ø Identify, procure & qualify equipment to support the process

Tech Transfer Execution n Conduct Gap analysis & Identify Gaps between originating and receiving facilities n Process/Method Gaps n n Information Gaps n n Conduct studies & generate data to support process / method Gather / Source technical documents to support process / method Facility Gaps n Process change request through appropriate capital approval committee n Gap Analysis yields Work packages n Work packages comprise Accountability Tables 39

Technology Transfer Execution Develop TT Plan Integrates action items from gap analysis with project timeline Directs completion of Work Packages from gap analysis Integrates validation plans with technology transfer 40

Technology Transfer Plan Elements of the TT Plan n n n n Strategic Overview Technical Descriptions Experimental Outline Validation Approach Success criteria Gates (accountability table) Training requirements Milestones & deliverables (schedule) TT Structure implements the plan 41

Functional Team Participation – Critical Drivers n Originating and Receiving Responsibilities Defined & Agreed up front. n n Excellent Management of Interfaces n n Establish relationships Manage Relationships Maintain Relationships Use metrics to track TT progress n n n 42 Key Stake holder participation Open Communication Manage and Track Action Item closure on the TT Team to complete Gap Closure Relationship Management (Team) n On-time milestone tracking % Documents transferred / generated % Training Complete % Gaps closed

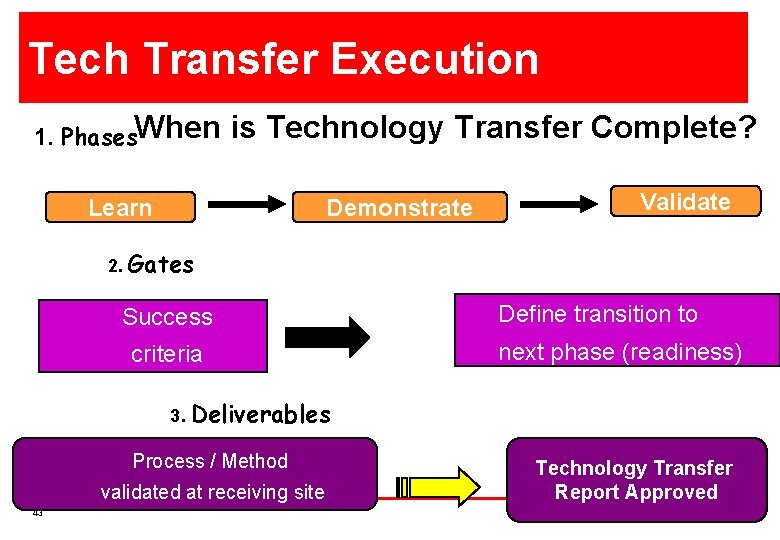
Tech Transfer Execution 1. Phases. When is Technology Transfer Complete? Learn Demonstrate Validate 2. Gates Success criteria Define transition to next phase (readiness) 3. Deliverables Process / Method validated at receiving site 43 Technology Transfer Report Approved

Tech Transfer Execution Phases of Technology Transfer § Phase I – Planning, Learning and Knowledge Capture n n Phase II – Demonstration Batch Phase n n n Training of SMEs at originating site Integration with originating/receiving site schedules Transfer of Technical Documentation to receiving site Transfer Execution at scale at receiving site Demonstration of robust process/method at receiving site Phase III – Validation Batch Phase n n Validation of process at receiving site Production of commercial material Gates are defined by the TT Plan 44

Functional Team Participation – Critical Drivers Technology Transfer SME Model Key individuals on “assignment” for training at originating site during learning phase n Gather Data & Source Documentation n Gain Expertise – learn “folklore” n Train Operations (customer) n Escort the process to Receiving site (Demo phase) n Trouble-shoot & support start-up (Validation phase) n 45

One-Team culture DO n n n 46 n Invest in face to face meetings Spend time in partner facilities (walk in their shoes) Assign specific SME partnerships Agree clear accountability and decision-making Constantly adapt to schedule opportunities Adopt formal, planned DON’T n n n Escalate everythingkeep the team empowered Allow ‘us and them’ to develop Keep critical information from team members

Vehicles for Team Success PEOPLE n n 47 Effort, commitment and expertise from teams (and assignees where available) Willingness to look for and exploit new ways to do things Resources - effective hiring ramp-up providing staff when needed and allowing training to occur Flexible working environment ORGANISATION n n n Focus on empowered teams with clear goals, accountabilities and timelines Effective integration and leveraging of external (business partner) staff A Quality organisation that acts as a partner to get the job done

Key Business Processes n Tech Transfer agreement n n n Tech Transfer plan and schedule n n n Resource loaded, part of overall project schedules Modified to meet threats and opportunities Defines Responsibilities Provides for experimental outline, validation approach & success criteria Change Control n n 48 Formal plan developed with input from donor and acceptor groups Provides clear governance, boundaries and deliverables Ensures process under transfer remain current (~ years) Impact on equipment shared promptly with design, construction, commissioning and validation

Management of Information Flow n Manage information received from Originating Site (technical / project) n n Preserve the integrity of the technical information during TT phases n n 49 Shared Servers – ‘E-Rooms’ Version Control, Transmittal Notices, Single Point Contacts Change Control (Multi-Site / Intra-Site) n Additional challenges during development & scale-up Data Registers – useful for trouble shooting Decision Registers & Change Registers – Track Changes during development & scale-up Gating Reports – captures findings from a TT phase

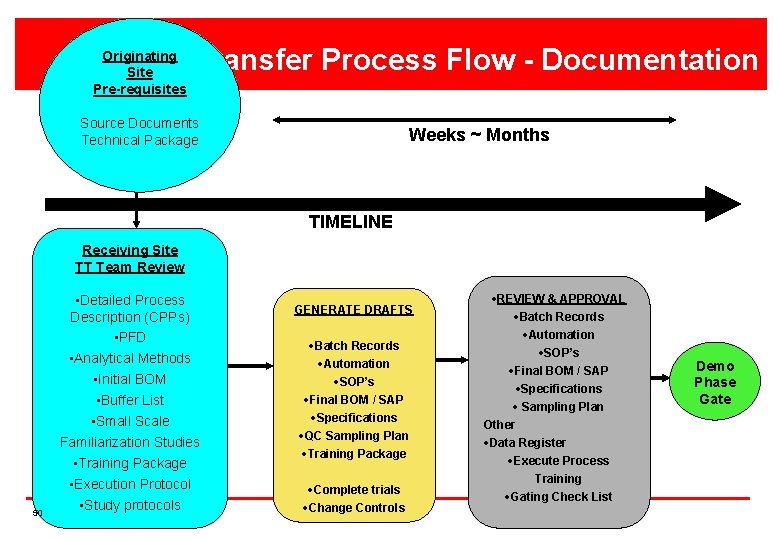
Tech Transfer Process Flow - Documentation Originating Site Pre-requisites Source Documents Technical Package Weeks ~ Months TIMELINE Receiving Site TT Team Review 50 • Detailed Process Description (CPPs) • PFD • Analytical Methods • Initial BOM • Buffer List • Small Scale Familiarization Studies • Training Package • Execution Protocol • Study protocols GENERATE DRAFTS ·Batch Records ·Automation ·SOP’s ·Final BOM / SAP ·Specifications ·QC Sampling Plan ·Training Package ·Complete trials ·Change Controls ·REVIEW & APPROVAL ·Batch Records ·Automation ·SOP’s ·Final BOM / SAP ·Specifications · Sampling Plan Other ·Data Register ·Execute Process Training ·Gating Check List Demo Phase Gate

Tech Transfer Completion Outcomes / Deliverables 1. Technology Transfer Package § Process / Method descriptions § Supporting Technical documentation 2. Technology Transfer Report Gaps closed n Training complete n Gating checklists for each phase complete n Process Validated n 51

Measure TT Success - Metrics Measurement of data, value, timeframe, numbers Number of Deviations n Operator or method errors n Equipment failure n Lots on hold n Number of investigations n Lots requiring rework n Equipment malfunction n 52

Measure TT Success - KPIs n Key Performance Indicators (KPIs) n n n n 53 Normalizing a few metrics so that efficiencies or effectiveness can be compared Number of lots required to complete validation Vs target Number of revalidations (target = 0) Number of lots failed in 100 lots Cycle time against target Yields against target Percent reworks (target = 0)

QUESTIONS? ? ? n 54 clement. farrar@gmail. com

Take 10 minutes! 55