Site Care Site Care Education Goals Review normal


































































- Slides: 77

Site Care

Site Care Education Goals • Review normal reactions after vaccination • Provide site care instructions (oral and written) • Provide contact information for concerns

Site Care Education Goals (2) • Provide successful vaccination “take” reading date and location information

Vaccination Site Reaction Day 4 Day 7 Day 14 Day 21

Normal, expected local reactions Usually seen ~1 week after vaccination • Soreness at vaccination site • Intense erythema surrounding the vaccination site • Lymphadenopathy (local): 25% – 50%

Normal, expected systemic symptoms – usually occur ~1 week after vaccination • • • Headache Myalgia Chills Nausea Fatigue 0. 3 – 37. 0%

Normal, expected systemic symptoms usually seen ~1 week after vaccination • Malaise • Fever – 100º F: 17% – 101º F: 7% – 102º F: 1. 4%

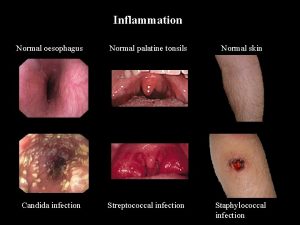
Normal variant responses & symptoms • • • Local satellite lesions Lymphangitis Local edema Viral cellulitis Mild rashes

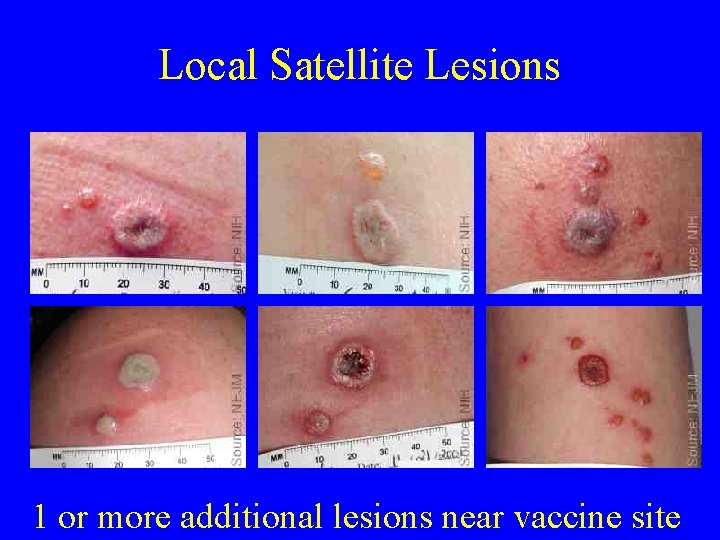
Local Satellite Lesions 1 or more additional lesions near vaccine site

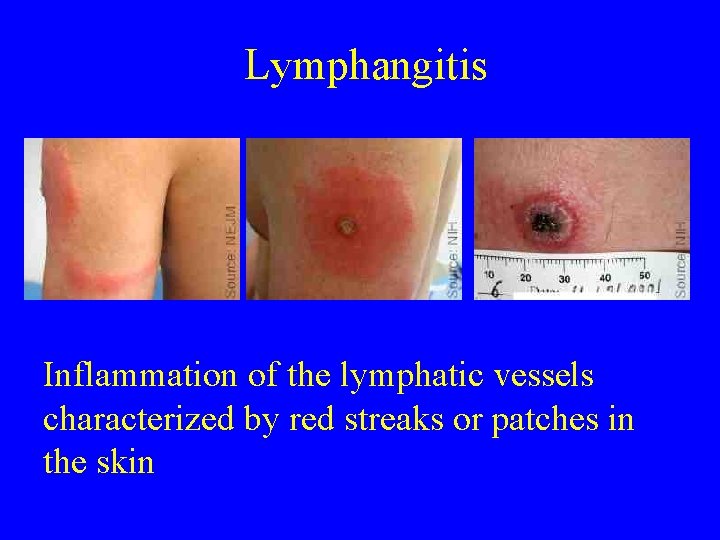
Lymphangitis Inflammation of the lymphatic vessels characterized by red streaks or patches in the skin

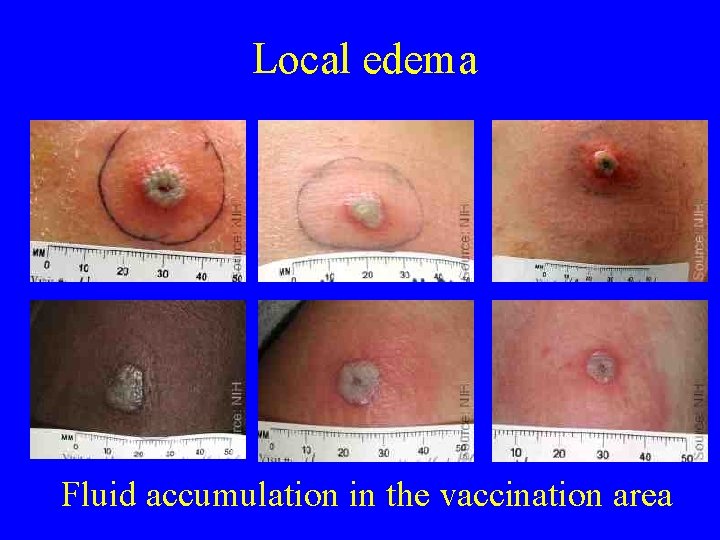
Local edema Fluid accumulation in the vaccination area

Site Care Clinical Issues • Vaccinia virus may be cultured from the site of a vaccination from 2 -3 days after vaccination until the scab separates • Care must be taken to prevent the spread of the virus to other parts of the body or other persons

Dressing • Keep covered with 2 x 2 gauze and tape at all times until scab separates • Semi-permeable occlusive bandage over 2 x 2 dressing for HCW at work • Use waterproof covering during shower

Dressing Changes • Wash hands with soap and water before and after every contact with the vaccination site or any materials (dressing, clothing, etc). • Change dressing every 1 -2 days

Dressing Changes (2) • HCW may change own dressing at home • Place used dressing in zip bag and discard in trash • Vary position of tape over gauze

Comfort Measures • Acetaminophen and ibuprofen for pain • DO NOT scratch—may use oral antihistamines for itching • DO NOT apply anything (ointments, salves or antibiotic band-aids) to the vaccine site • DO NOT apply heat or cold to the vaccine site

At-work Issues • Cover dressing with semi-permeable membrane, e. g. , Opsite • Wear long-sleeves over dressing • HCW do not need to be furloughed • Have dressing assessed on work days before beginning shift

At-home Issues • Use normal laundering (hot water) to wash clothes, towels or sheets that have touched the vaccination site • Keep family members from touching your vaccination site (keep site covered)

At-home Issues (2) • Wear long sleeves to bed • Wash hands first thing in the morning • Dispose of scab in zip bag and discard in trash

Additional Clinic Roles

Vaccination Assistants • May assist vaccine administrator with all aspects of pre and post vaccination activities • May set up/break down vaccination stations

Vaccination Assistants (2) • May assure area is available for vaccinees having reactions • May replenish vaccine station supplies • May apply bandages to vaccination site

Data Entry Staff • MDCH intends to provide data entry staff • Vaccination not required • Data entry discussion to follow

Entry/Exit Monitors • Maintain clinic flow • May answer questions related general vaccine clinic operations (entrances, exits, parking, bathroom locations etc. ) • May be clerical staff or volunteers • Vaccination not needed

Security • Assure clinic parking is adequate, close and protected • Provide telephone numbers for police, fire, utilities, facility owner/manager

Security (2) • Assure facility is secure, well lighted and functional • Vaccination not indicated

Supply Issues

Supplies Provided by MDCH • 4 X 4 Tegederms • $50. 00 per vaccinee for other supplies • Copies of CDC vaccination packets (as requested in January by each region) • Color brochures for each vaccinee to be handed out during site care instruction

Supplies Provided by MDCH • Vaccination card for vaccinee • Vaccine Information Statements • Consent forms

Additional Clinic Supplies • Detailed supply checklist provided in Handout #4

Section V: Issues Data

Data System • MCIR – Modifications: age restrictions, assessment, adverse event tracking, cost, time to develop, and daily extract to CDC of the data – May be available for Stage 2

Data System • Pre-Event Vaccination System (PVS) – Written by CDC – Web-based application – Digital certificates used for security

Smallpox Vaccination Form— Data Flow • Matches clinic flow • Printed in triplicate • Top sheet used for data entry and sent to MDCH • Second sheet sent to clinic responsible for reading the “Take” response • Third sheet given to the vaccinee

Greeter & Smallpox Vaccination Form • Greeter gives form to vaccinee • Vaccinee completes – Patient Information – Vaccination History

Nurse Screener & the Form • The nurse screener completes – Referring Organization – Current Vaccination Questions – Disposition once the patient signs the form giving consent to be vaccinated • Record the PVN on each copy of the form

Vaccinator/Vaccination Assistant & the Form • After vaccination, nurse vaccinator or vaccination assistant will – Document the date of administration and sign the form – Fill in the vaccine, diluent and batch information

Vaccinator/Vaccination Assistant & the Form (2) • After vaccination, nurse vaccinator or vaccination assistant will – Complete the vaccine record card for vaccinees to have for their records

Site Care Educator & the Form • Site care educator will record the date and location where the “take” response should be read

Data Entry Staff • The completed form is then given to the data entry staff for entry into the PVS

“Take” Response Readers • The “take” response should be recorded in the appropriate section at the bottom of the form • The form will be sent to MDCH for data entry

“Take” Response Readers (2) • The “take” response should also be recorded on the vaccinee’s vaccine record card • An additional form will be developed for use at sites where the “take” response will be read in case the original form is not available

Data Reminders • Use of the PVN is key to tracking the individual and linking any follow-up activity back to the same individual • MDCH plans to supply staff for data entry at clinics depending on the number of clinics occurring simultaneously

Data Reminders (2) • CDC expects us to account for every dose of vaccine administered and have a “take” reading done on every person receiving the vaccine

Section VI: Evaluating Vaccine “Take”

Evaluating Vaccination Site for “Take” • Major response • Equivocal response • No response

Major “Take” Response

Major “Take” Response (2) • Need to see one of the following 6 -10 days after vaccination: – Clear-cut pustule OR – Area of definite induration or congestion around a central lesion, e. g. , an ulcer or a scab

Equivocal Response • Any other reaction • May result from – A person being sufficiently immune to suppress viral replication – Sub-potent vaccine or improper technique

Equivocal Reaction (2) • May result from (cont. ) – Hypersensitivity reaction to components of the vaccine • Do not confuse with reaction to tape

No Response • No signs of major or equivocal reaction to vaccine

Re-vaccination • Indicated for – Equivocal response – No response • Vaccination repeated using vaccine from another vial when possible

Re-vaccination (2) • After 2 vaccinations without major reaction, seek medical consultation • O. K. to re-vaccinate in same (nondominant) arm

Coordinating “Take” Evaluation for HCRT • Regional vaccination coordinator and hospital site facility coordinator identify a plan to evaluate “takes” • Plan will need to be communicated to the vaccination clinic manager • “Take” response reading date must be given to each vaccinee

Coordinating “Take” Evaluation for PHRT • Regional vaccination coordinator identifies plan to evaluate “takes” for public health staff • Plan communicated to PHRT members • “Take” response reading date given to each vaccinee

“Take” Evaluation Process • Vaccine “takes” evaluated by someone trained in “take” evaluation • Schedule “take” evaluation 6 -10 days after vaccination • Record vaccine “take” on form & on vaccinee’s vaccination card

“Take” Evaluation Process (2) • Employer follow-up is needed for vaccinees who miss appointments for “take” evaluation • Refer for medical consultation after 2 unsuccessful “takes”

Section VII: Reporting Adverse Events

True Adverse Reactions • • Focus for monitoring Rare Can be serious May require specific therapy

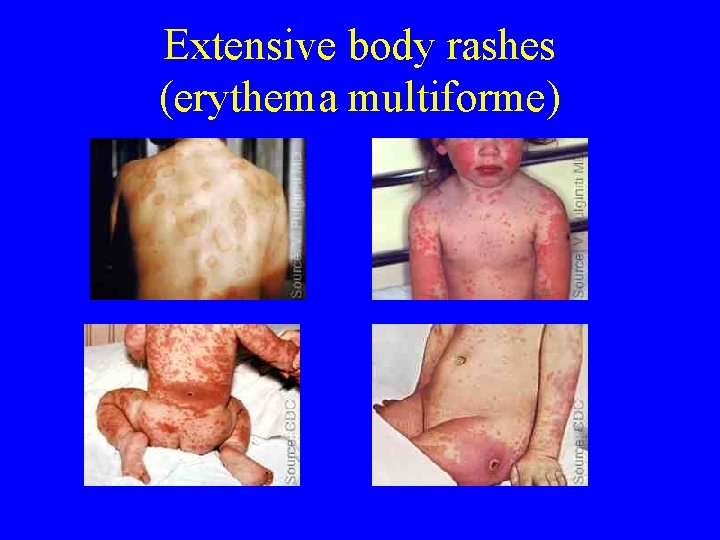
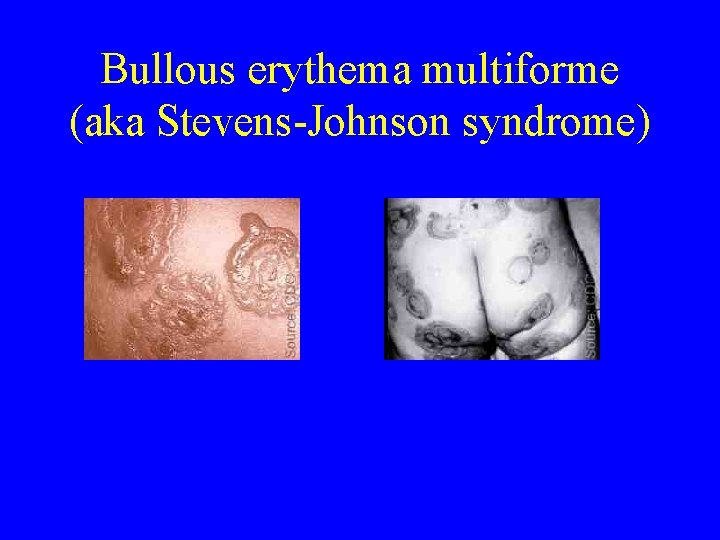
True adverse reactions • Extensive body rashes (erythema multiforme) • Bullous erythema multiforme • Bacterial infection of site • Inadvertent autoinoculation • Contact inoculation

True adverse reactions (2) • • Generalized vaccinia Eczema vaccinatum Progressive vaccinia Post-vaccinial Encephalitis

Extensive body rashes (erythema multiforme)

Bullous erythema multiforme (aka Stevens-Johnson syndrome)

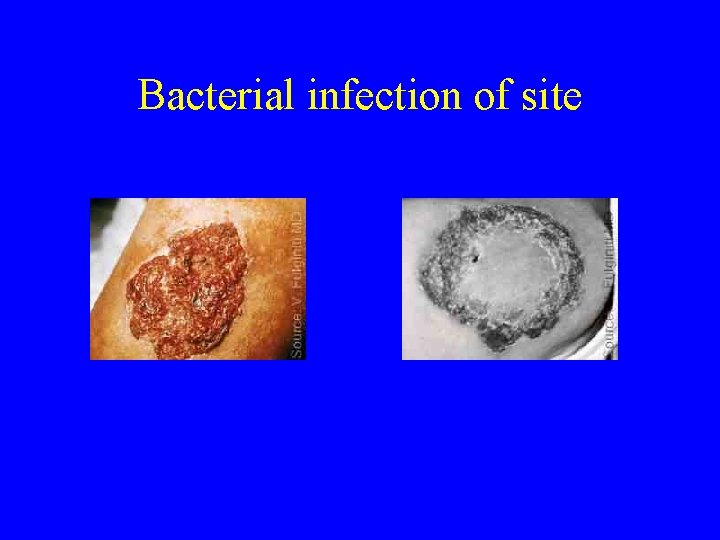
Bacterial infection of site

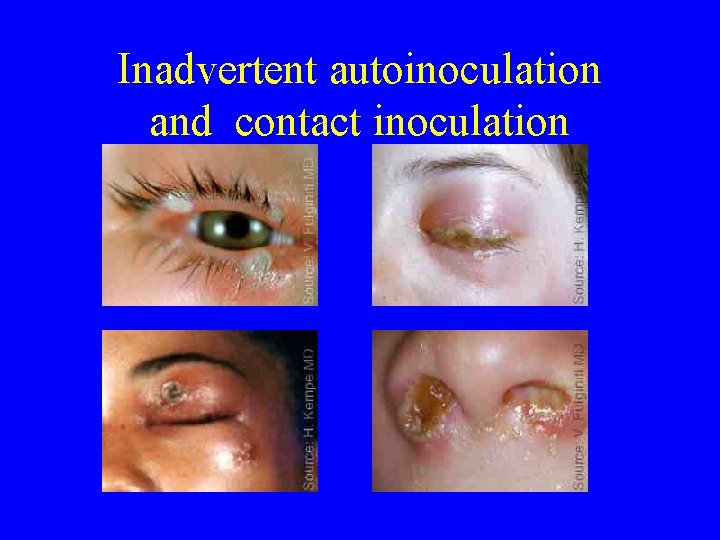
Inadvertent autoinoculation and contact inoculation

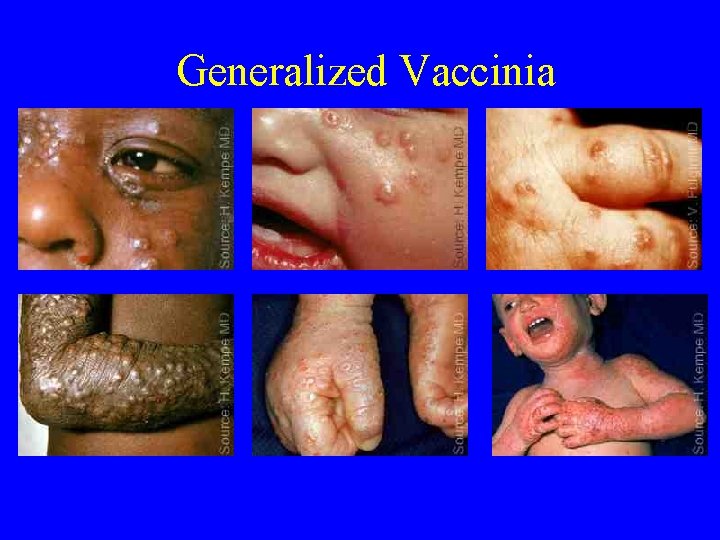
Generalized Vaccinia

Eczema vaccinatum

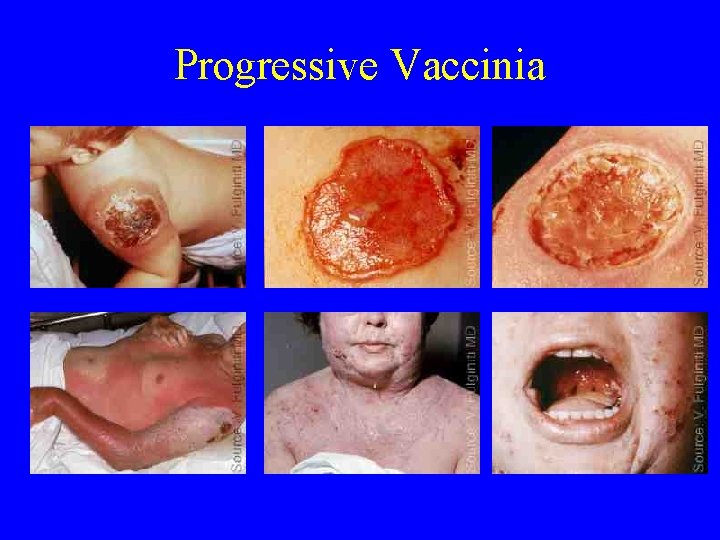
Progressive Vaccinia

Post-Vaccinial Encephalitis • Rare • Believed to result from autoimmune or allergic reaction • No specific therapy • VIG is not effective and is not recommended

Management and Reporting: Role of the HAP • Designated “Hospital-Assigned Physician” • Evaluates and manages reactions • Consults with state and national resources as necessary • Reports adverse reactions to VAERS • Indicates PVN on VAERS report

Adverse Events Summary • Some local & systemic reactions are common & expected • Variations of normal “takes” occur • Some responses are dramatic, but selflimited

Adverse Events Summary (2) • Severe reactions can occur but are rare • Monitoring to focus on serious reactions • Careful screening can minimize occurrence of serious reactions

Section VIII: MDCH Role

MDCH Role • Assistance with training as requested • Assistance with data system and data issues • Assistance with evaluating adverse events

Questions? • Questions may be – Faxed to 517/335 -9855 – E-mailed to fasanon@michigan. gov

For More Information… • Additional smallpox information available at – http: //www. michigan. gov/mdch – http: //www. bt. cdc. gov

Michigan Dept of Community Health P. O. Box 30195 Lansing, MI 48909 517/335 -8159 517/335 -9855 (fax) Contact: Nancy Fasano, fasanon@michigan. gov http: //www. michigan. gov/mdch
 Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals General goals and specific goals
General goals and specific goals Motivation in consumer behaviour
Motivation in consumer behaviour Literature review goals
Literature review goals Levels of health care primary secondary tertiary
Levels of health care primary secondary tertiary Hot site cold site warm site disaster recovery
Hot site cold site warm site disaster recovery Multicultural education characteristics and goals
Multicultural education characteristics and goals Goals of mathematics education in the philippines
Goals of mathematics education in the philippines Smart goals for special education administrators
Smart goals for special education administrators Goals for education
Goals for education Goals of science teaching
Goals of science teaching Goals of multicultural education
Goals of multicultural education It's normal to be normal
It's normal to be normal Goals of care alberta chart
Goals of care alberta chart Shared goals of care
Shared goals of care Nursing diagnosis for grieving
Nursing diagnosis for grieving Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Uncontrollable spending ap gov
Uncontrollable spending ap gov Narrative review vs systematic review
Narrative review vs systematic review Traditional and systematic review venn diagram
Traditional and systematic review venn diagram Narrative review vs systematic review
Narrative review vs systematic review Cbc manitoba education review
Cbc manitoba education review Cariotipul uman normal
Cariotipul uman normal Care first counselling review
Care first counselling review Chapter 3 career in health care
Chapter 3 career in health care Difference between als and formal education
Difference between als and formal education Differences between health promotion and health education
Differences between health promotion and health education Backbone of extension education
Backbone of extension education Certified podortho nurse
Certified podortho nurse Example of nursing process
Example of nursing process Early childhood education and care directorate
Early childhood education and care directorate Early education workforce registry
Early education workforce registry Six bricks care for education
Six bricks care for education Workforce registry ca
Workforce registry ca How to become a lawn care specialist
How to become a lawn care specialist Standard 3 care certificate
Standard 3 care certificate Magnetii sunt corpurile care
Magnetii sunt corpurile care Palliative care versus hospice care
Palliative care versus hospice care Cum se înmulțesc animalele
Cum se înmulțesc animalele Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Care certificate 10
Care certificate 10 Hip fracture clinical care standard
Hip fracture clinical care standard Care value base health and social care
Care value base health and social care Iv placement sites
Iv placement sites Ribosome epa site
Ribosome epa site Site analysis and inventory
Site analysis and inventory Untangle vpn site to site
Untangle vpn site to site Workshop goals examples
Workshop goals examples Work immersion example
Work immersion example Women's ministry goals and objectives
Women's ministry goals and objectives Women's ministry goals and objectives
Women's ministry goals and objectives Lenovo mission statement
Lenovo mission statement School readiness goals
School readiness goals Footloose industry definition ap human geography
Footloose industry definition ap human geography Smart goals avid
Smart goals avid Warehouse objectives
Warehouse objectives Perspective taking goals
Perspective taking goals Universal health care meaning
Universal health care meaning What are your long term goals examples
What are your long term goals examples Objective of triage
Objective of triage What is tpcast
What is tpcast What evidence do you see of tojo being militaristic
What evidence do you see of tojo being militaristic Nursing process order
Nursing process order Thalidomide
Thalidomide The practice of public relations fraser p. seitel pdf
The practice of public relations fraser p. seitel pdf Ponyboy goals
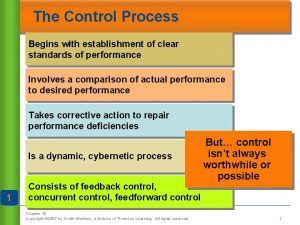
Ponyboy goals A control process begins with
A control process begins with How are conflicts among economic goals resolved?
How are conflicts among economic goals resolved? Big picture learning goals
Big picture learning goals Lem goals
Lem goals Teacher goals for 2020-2021
Teacher goals for 2020-2021 Goals and objectives examples
Goals and objectives examples Structure of counselling process
Structure of counselling process Amt learning goals
Amt learning goals Chapter 7 learning goals outline sociology
Chapter 7 learning goals outline sociology Passionate love ap psychology definition
Passionate love ap psychology definition Superordinate goals psychology definition
Superordinate goals psychology definition