Single Replacement Reactions Silver replacing Copper in reaction


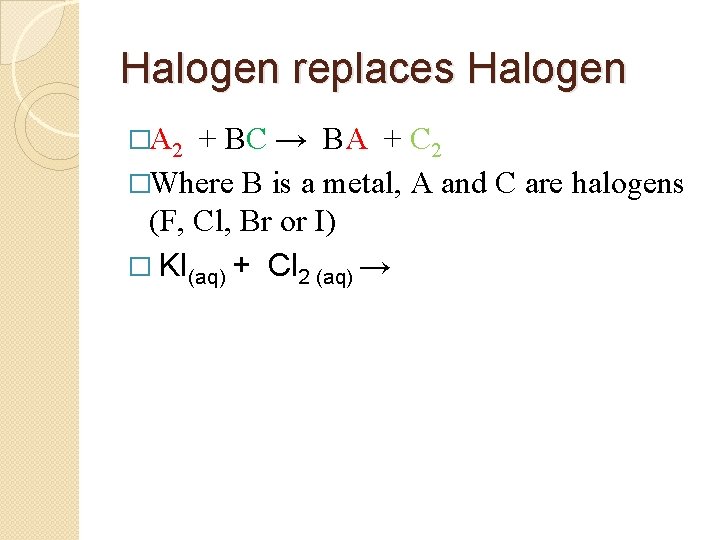
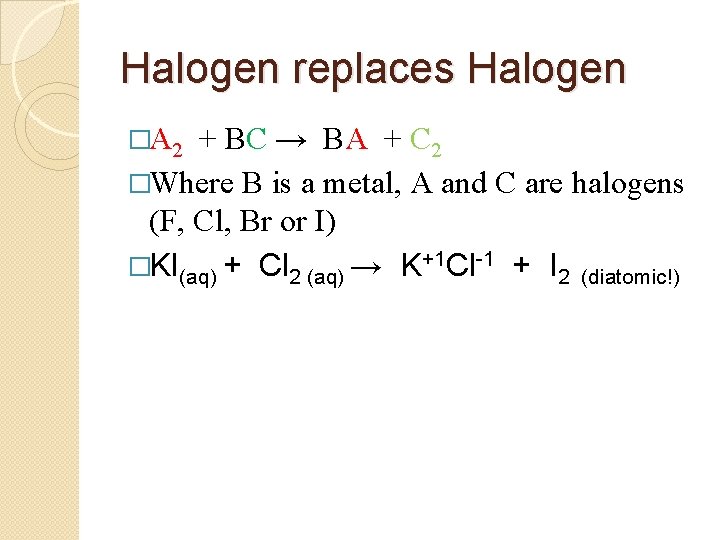
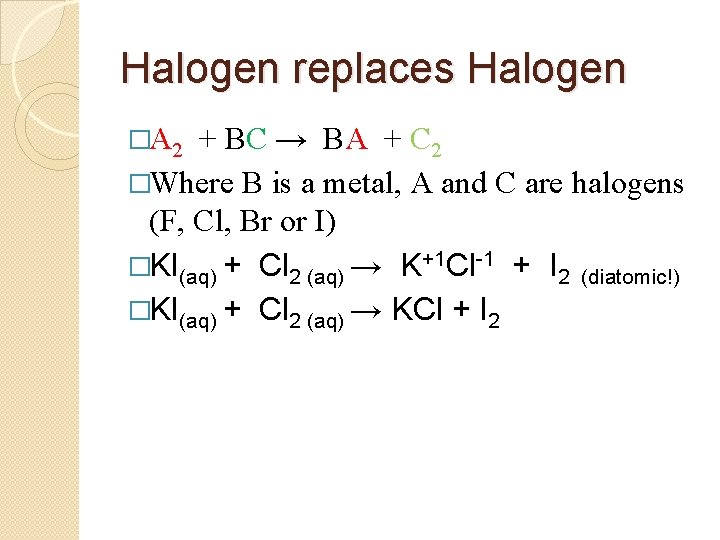


- Slides: 18

Single Replacement Reactions Silver replacing Copper in reaction of silver nitrate and copper Link to silver nitrate and copper

“Like replaces Like” � Element �Metals + Compound → Compound + Element replace metals and halogens replace halogens. �MA + MBC → MAC + MB �Where MA and MB are metals, C is a nonmetal or negative polyatomic ion. �X 2 + MY → MA + Y 2 �Where M is a metal, X and Y are halogens (F, Cl, Br or I) �Link to Mc. Graw-Hill tutorial

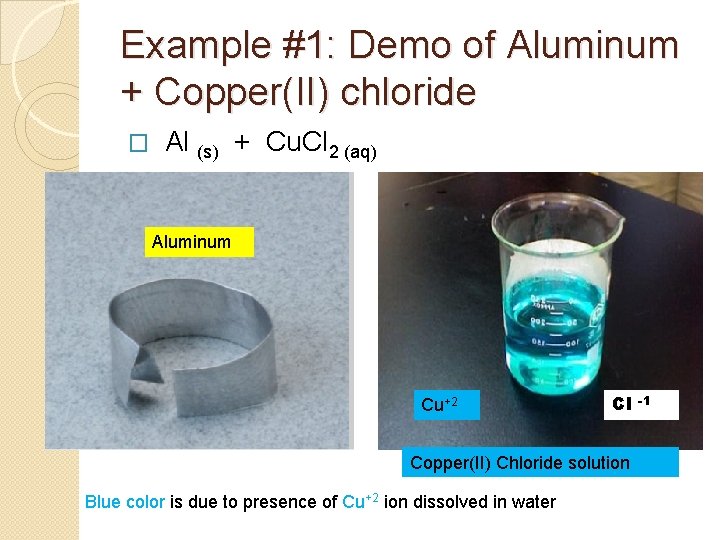
Example #1: Demo of Aluminum + Copper(II) chloride Al (s) + Cu. Cl 2 (aq) → � Aluminum Cu+2 Cl Copper(II) Chloride solution Blue color is due to presence of Cu+2 ion dissolved in water -1

What is the reddish solid that formed? Why has the solution turned from blue to colorless? Immediately after mixing Final appearance after 30 minutes

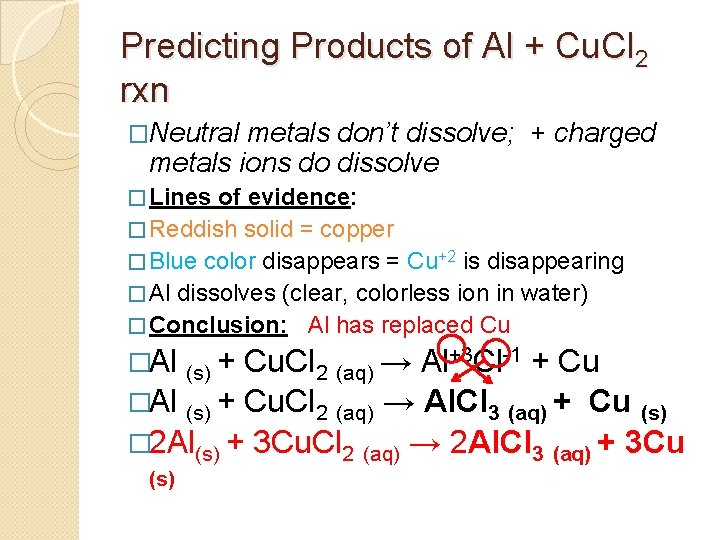
Predicting Products of Al + Cu. Cl 2 rxn �Neutral metals don’t dissolve; + charged metals ions do dissolve � Lines of evidence: � Reddish solid = copper � Blue color disappears = Cu+2 is disappearing � Al dissolves (clear, colorless ion in water) � Conclusion: Al has replaced Cu �Al (s) + Cu. Cl 2 (aq) →

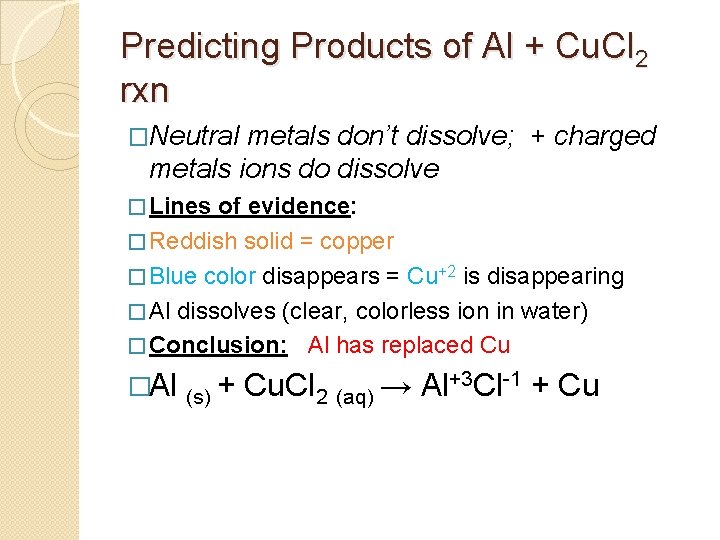
Predicting Products of Al + Cu. Cl 2 rxn �Neutral metals don’t dissolve; + charged metals ions do dissolve � Lines of evidence: � Reddish solid = copper � Blue color disappears = Cu+2 is disappearing � Al dissolves (clear, colorless ion in water) � Conclusion: Al has replaced Cu �Al (s) + Cu. Cl 2 (aq) → Al+3 Cl-1 + Cu

Predicting Products of Al + Cu. Cl 2 rxn �Neutral metals don’t dissolve; + charged metals ions do dissolve � Lines of evidence: � Reddish solid = copper � Blue color disappears = Cu+2 is disappearing � Al dissolves (clear, colorless ion in water) � Conclusion: Al has replaced Cu �Al (s) + Cu. Cl 2 (aq) → Al+3 Cl-1 + Cu Cu. Cl 2 (aq) → Al. Cl 3 (aq) + Cu (s) � 2 Al(s) + 3 Cu. Cl 2 (aq) → 2 Al. Cl 3 (aq) + 3 Cu (s)
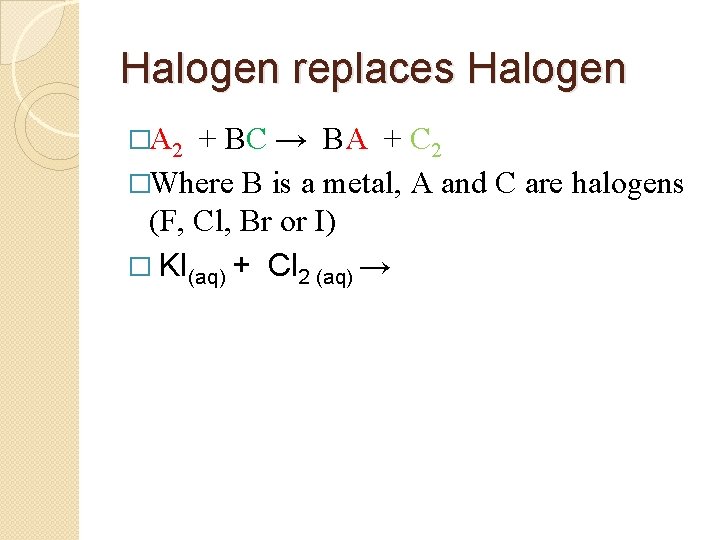
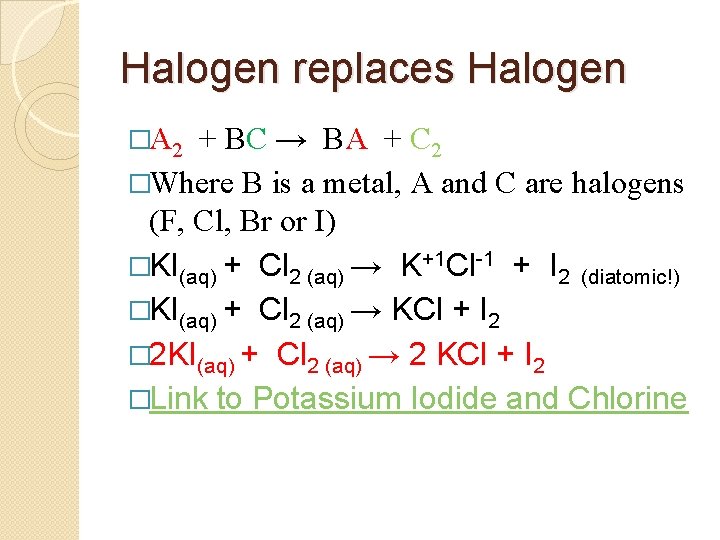
Halogen replaces Halogen �A 2 + BC → BA + C 2 �Where B is a metal, A and C are halogens (F, Cl, Br or I) � KI(aq) + Cl 2 (aq) →
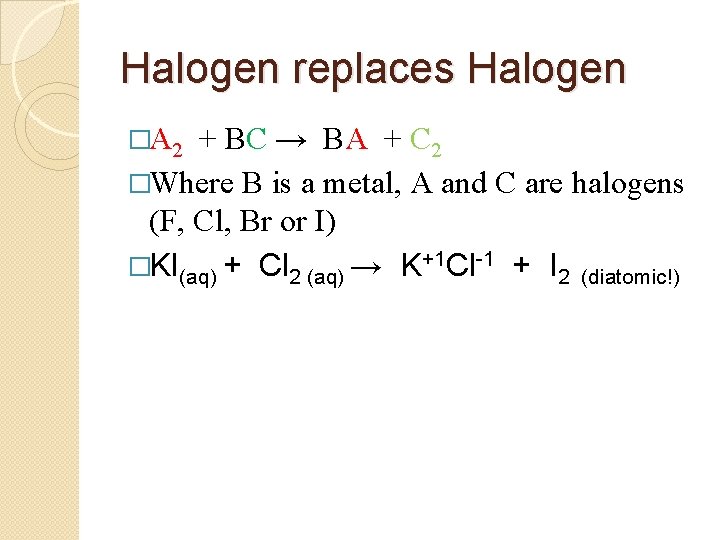
Halogen replaces Halogen �A 2 + BC → BA + C 2 �Where B is a metal, A and C are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) → K+1 Cl-1 + I 2 (diatomic!)
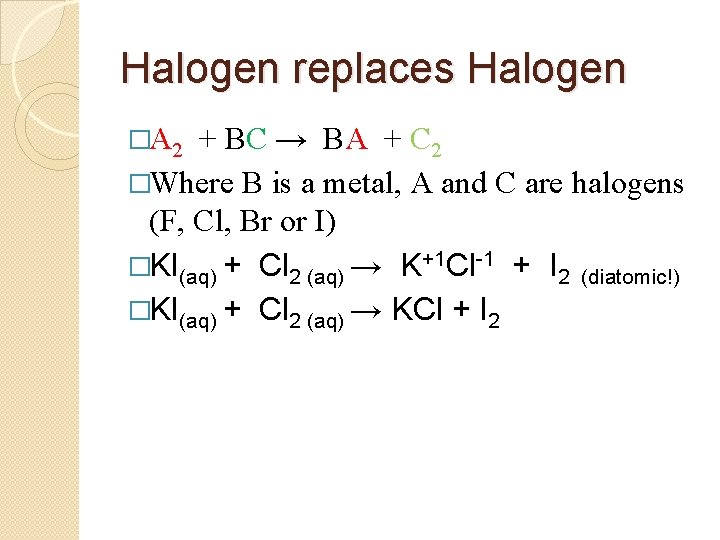
Halogen replaces Halogen �A 2 + BC → BA + C 2 �Where B is a metal, A and C are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) → K+1 Cl-1 + I 2 (diatomic!) �KI(aq) + Cl 2 (aq) → KCl + I 2

Halogen replaces Halogen �A 2 + BC → BA + C 2 �Where B is a metal, A and C are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) → K+1 Cl-1 + I 2 (diatomic!) �KI(aq) + Cl 2 (aq) → KCl + I 2 � 2 KI(aq) + Cl 2 (aq) → 2 KCl + I 2 �Link to Potassium Iodide and Chlorine

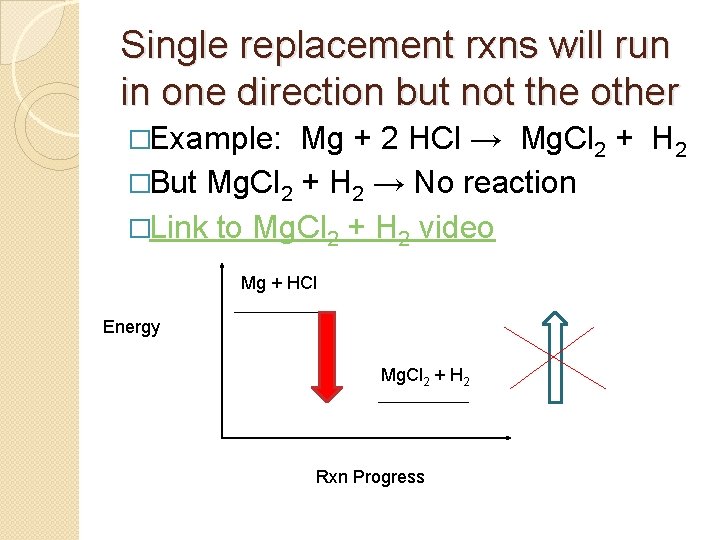
Activity Series �Some processes are spontaneous in one direction but not the other � Example: Rock can fall from a cliff to the ground, but a rock on the ground can’t climb the cliff by itself.

Single replacement rxns will run in one direction but not the other �Example: Mg + 2 HCl → Mg. Cl 2 + H 2 �But Mg. Cl 2 + H 2 → No reaction �Link to Mg. Cl 2 + H 2 video Mg + HCl Energy Mg. Cl 2 + H 2 Rxn Progress

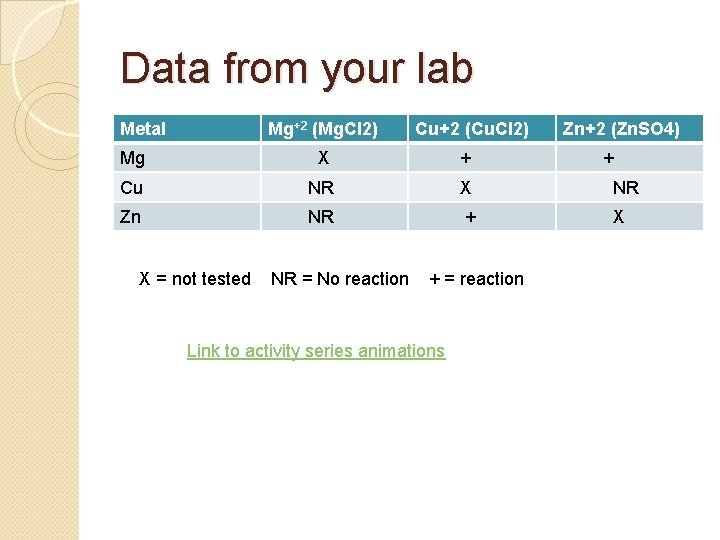
Data from your lab Metal Mg+2 (Mg. Cl 2) Cu+2 (Cu. Cl 2) Zn+2 (Zn. SO 4) Mg X + Cu NR X NR Zn NR + X X = not tested NR = No reaction + = reaction Link to activity series animations +

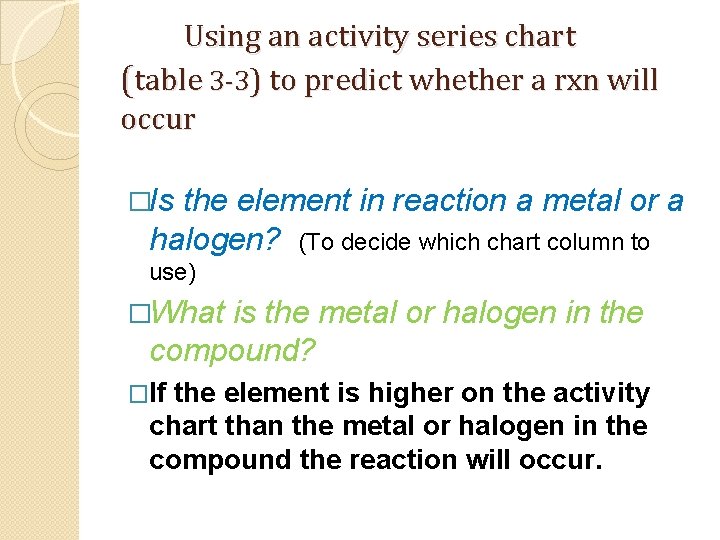
Using an activity series chart (table 3 -3) to predict whether a rxn will occur �Is the element in reaction a metal or a halogen? (To decide which chart column to use) �What is the metal or halogen in the compound? �If the element is higher on the activity chart than the metal or halogen in the compound the reaction will occur.

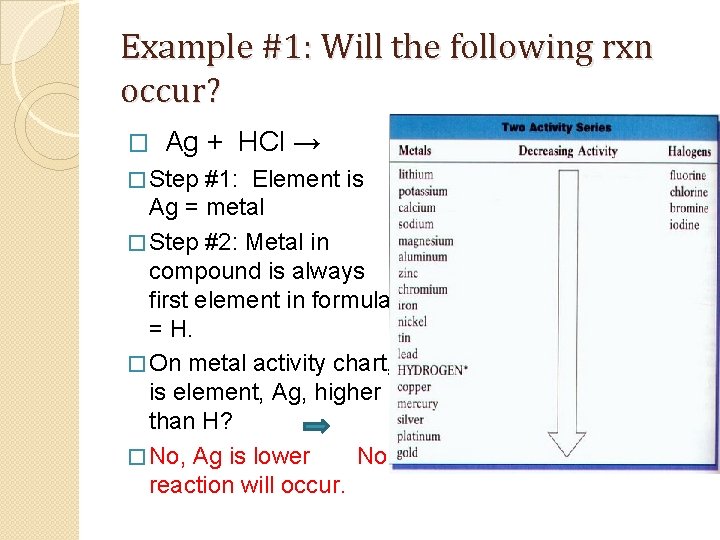
Example #1: Will the following rxn occur? � Ag + HCl → � Step #1: Element is Ag = metal � Step #2: Metal in compound is always first element in formula = H. � On metal activity chart, is element, Ag, higher than H? � No, Ag is lower No reaction will occur.

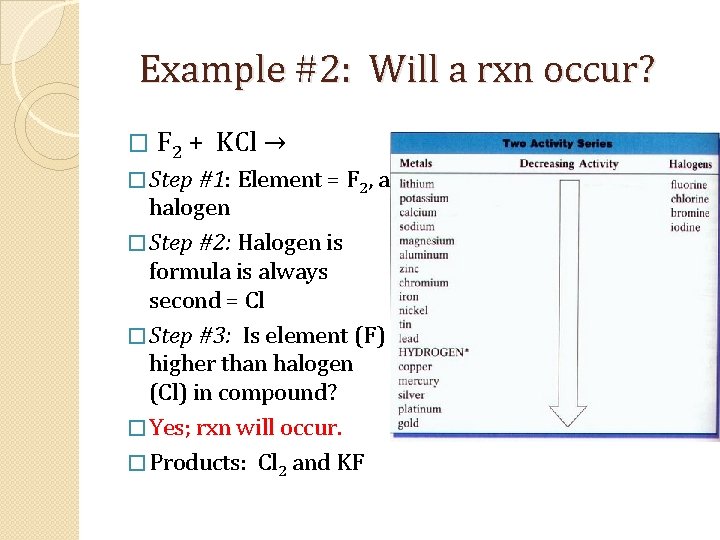
Example #2: Will a rxn occur? � F 2 + KCl → � Step #1: Element = F 2, a halogen � Step #2: Halogen is formula is always second = Cl � Step #3: Is element (F) higher than halogen (Cl) in compound? � Yes; rxn will occur. � Products: Cl 2 and KF

Applications of Activity Series: A more active metal being used to protect a metal supporting a structure � Using Zinc (more active) to protect iron (less chemically active but physically stronger); the iron inside the concrete is helping to support the weight of the bridge