Single Replacement Reactions Diatomic elementsdont forget For example




- Slides: 16

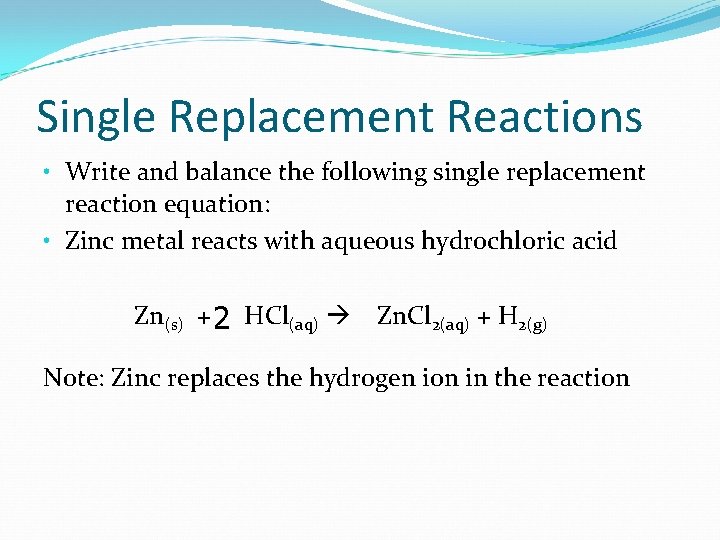
Single Replacement Reactions

Diatomic elements—don’t forget! For example, Oxygen is O 2 as an element. H 2, N 2, F 2, O 2, I 2, Cl 2, Br 2 Memorize this list!

Single replacement reactions

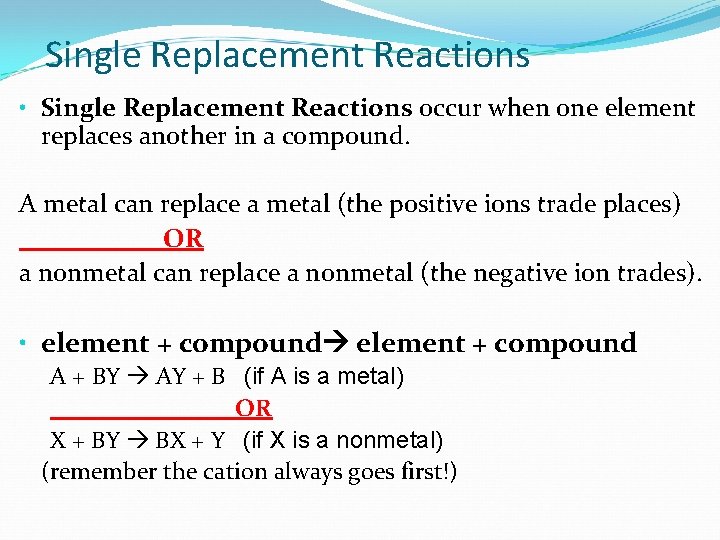
Single Replacement Reactions • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (the positive ions trade places) OR a nonmetal can replace a nonmetal (the negative ion trades). • element + compound A + BY AY + B (if A is a metal) OR X + BY BX + Y (if X is a nonmetal) (remember the cation always goes first!)

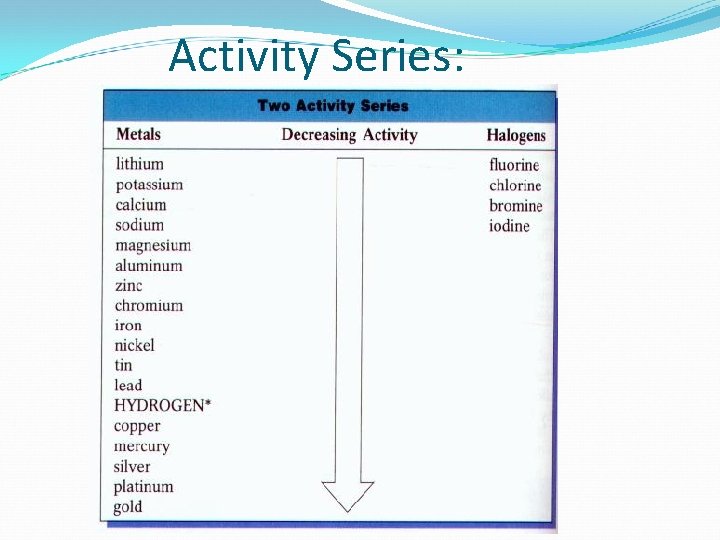
Activity Series:

Single Replacement Reactions

Single Replacement Reactions • Write and balance the following single replacement reaction equation: • Zinc metal reacts with aqueous hydrochloric acid Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) Note: Zinc replaces the hydrogen ion in the reaction

Single Replacement Reactions Sodium bromide solid reacts with chlorine gas 2 Na. Br(aq) + Cl 2(g) 2 Na. Cl(aq) + Br 2(l) Note that chlorine replaces bromine in the compound • Aluminum metal reacts with aqueous copper (II) nitrate Al(s)+ Cu(NO 3)2(aq) Copper DOES NOT reaction with aqueous aluminum nitrate. Copper is below aluminum on the activity series. Cu(s)+ Al(NO 3)3(aq) No Reaction

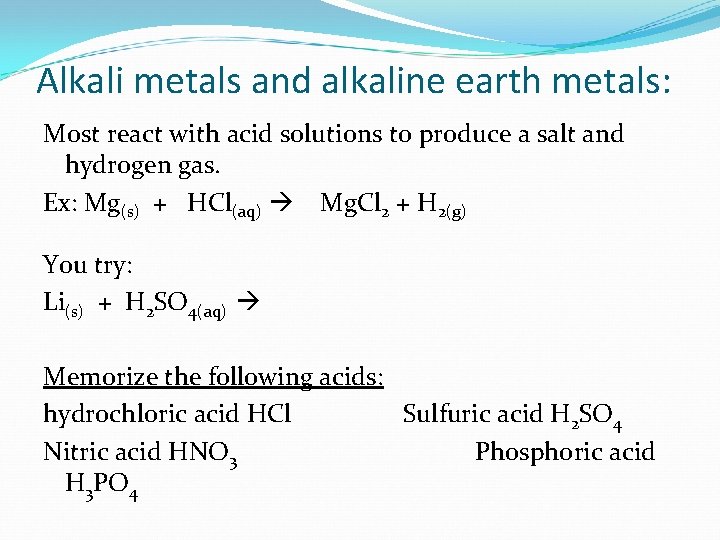
Alkali metals and alkaline earth metals: Most react with acid solutions to produce a salt and hydrogen gas. Ex: Mg(s) + HCl(aq) Mg. Cl 2 + H 2(g) You try: Li(s) + H 2 SO 4(aq) Memorize the following acids: hydrochloric acid HCl Sulfuric acid H 2 SO 4 Nitric acid HNO 3 Phosphoric acid H 3 PO 4

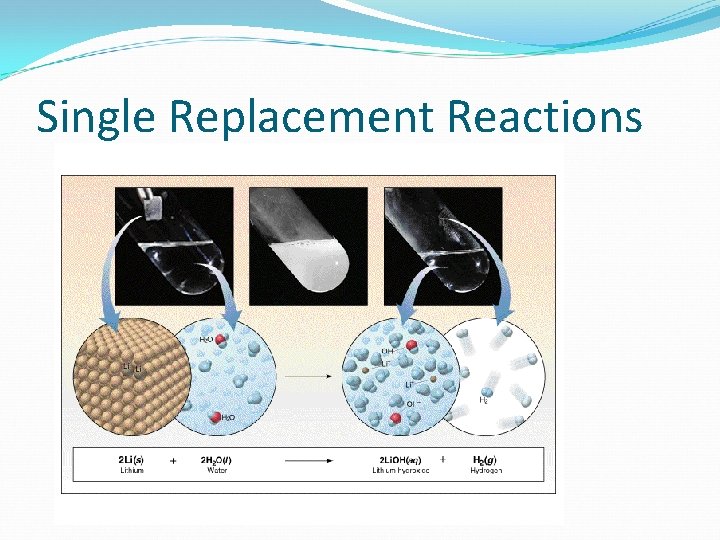
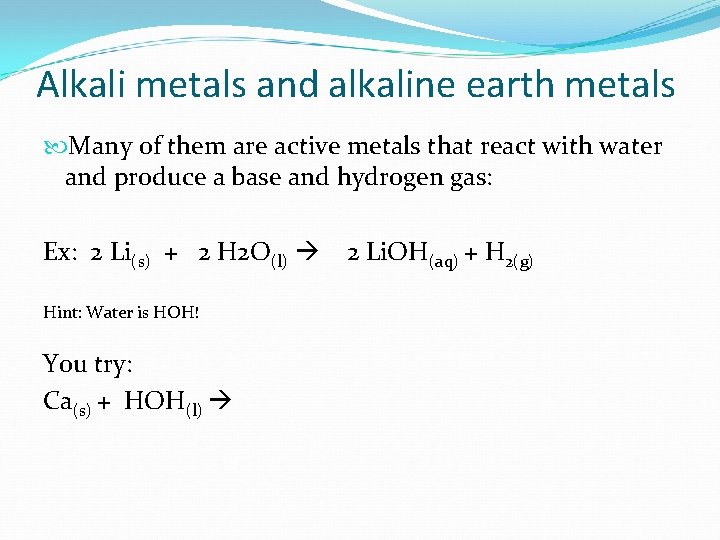
Alkali metals and alkaline earth metals Many of them are active metals that react with water and produce a base and hydrogen gas: Ex: 2 Li(s) + 2 H 2 O(l) Hint: Water is HOH! You try: Ca(s) + HOH(l) 2 Li. OH(aq) + H 2(g)

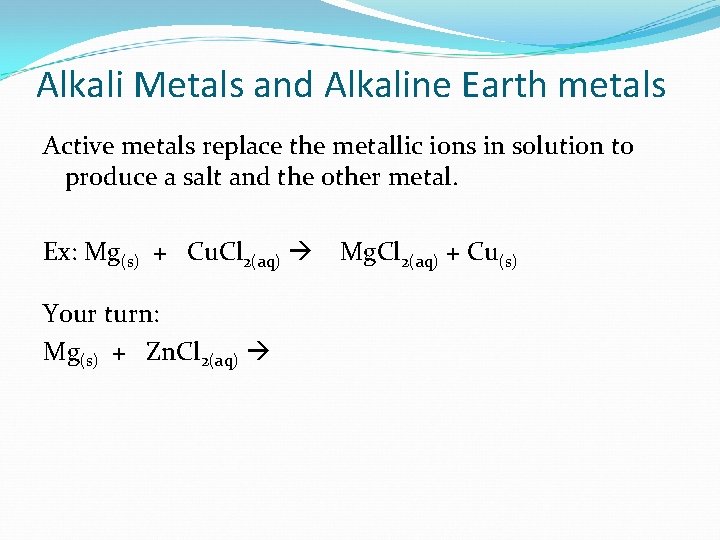
Alkali Metals and Alkaline Earth metals Active metals replace the metallic ions in solution to produce a salt and the other metal. Ex: Mg(s) + Cu. Cl 2(aq) Your turn: Mg(s) + Zn. Cl 2(aq) Mg. Cl 2(aq) + Cu(s)

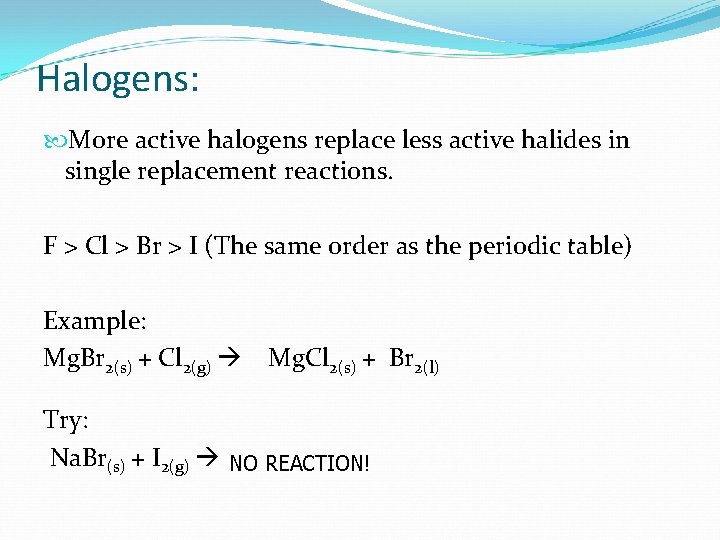
Halogens: More active halogens replace less active halides in single replacement reactions. F > Cl > Br > I (The same order as the periodic table) Example: Mg. Br 2(s) + Cl 2(g) Mg. Cl 2(s) + Br 2(l) Try: Na. Br(s) + I 2(g) NO REACTION!

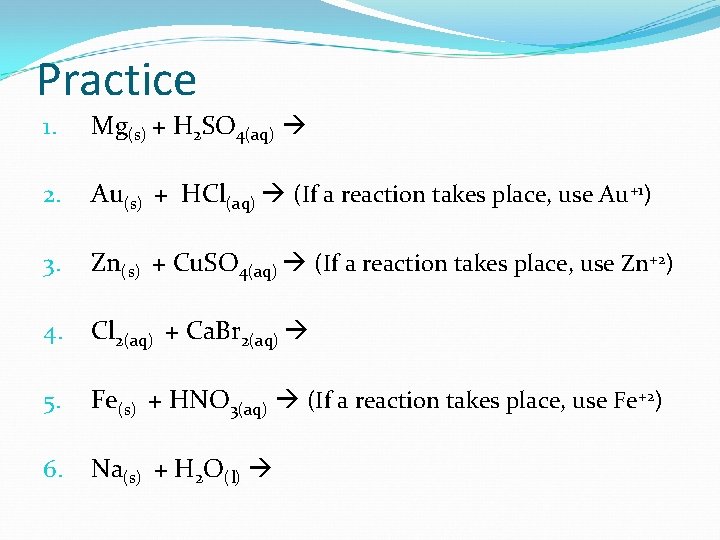
Practice 1. Mg(s) + H 2 SO 4(aq) 2. Au(s) + HCl(aq) (If a reaction takes place, use Au+1) 3. Zn(s) + Cu. SO 4(aq) (If a reaction takes place, use Zn+2) 4. Cl 2(aq) + Ca. Br 2(aq) 5. Fe(s) + HNO 3(aq) (If a reaction takes place, use Fe+2) 6. Na(s) + H 2 O(l)

Answers: Check your work! 1. Mg(s) + H 2 SO 4(aq) Mg. SO 4(aq) + H 2(g) 2. Au(s) + HCl(aq) No reaction 3. Zn(s) + Cu. SO 4(aq) Cu(s) + Zn. SO 4(aq) 4. Cl 2(aq) + Ca. Br 2(aq) + Ca. Cl 2(aq) 5. Fe(s) + 2 HNO 3(aq) H 2(g) + Fe(NO 3)2(aq) 6. 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g)

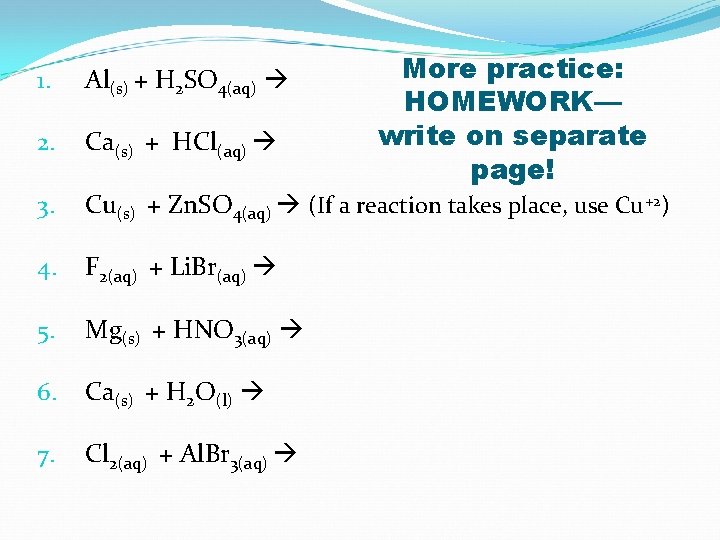
More practice: HOMEWORK— write on separate page! 1. Al(s) + H 2 SO 4(aq) 2. Ca(s) + HCl(aq) 3. Cu(s) + Zn. SO 4(aq) (If a reaction takes place, use Cu+2) 4. F 2(aq) + Li. Br(aq) 5. Mg(s) + HNO 3(aq) 6. Ca(s) + H 2 O(l) 7. Cl 2(aq) + Al. Br 3(aq)

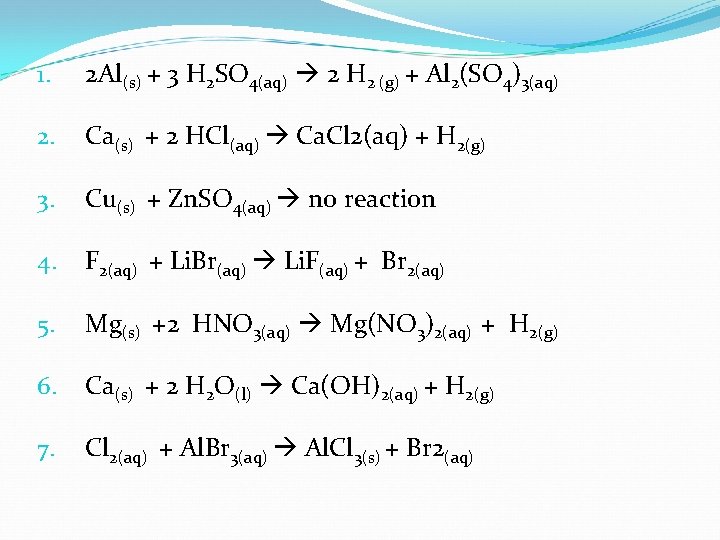
1. 2 Al(s) + 3 H 2 SO 4(aq) 2 H 2 (g) + Al 2(SO 4)3(aq) 2. Ca(s) + 2 HCl(aq) Ca. Cl 2(aq) + H 2(g) 3. Cu(s) + Zn. SO 4(aq) no reaction 4. F 2(aq) + Li. Br(aq) Li. F(aq) + Br 2(aq) 5. Mg(s) +2 HNO 3(aq) Mg(NO 3)2(aq) + H 2(g) 6. Ca(s) + 2 H 2 O(l) Ca(OH)2(aq) + H 2(g) 7. Cl 2(aq) + Al. Br 3(aq) Al. Cl 3(s) + Br 2(aq)