SINCE 2003 Pharmaceutical Quality Management Computerized System Validation

















- Slides: 34

SINCE 2003 Pharmaceutical Quality Management 자동화장치 관리 (Computerized System Validation) 한미약품㈜ 평택공단 세파플랜트 11 DEC. 2014

목차 1. 법적근거(Regulations & Guidelines) 2. 배경(Background) 3. 적용사례(Case study)



법적근거(Regulations & Guidelines) Ø EU GMP ü EU GMP Guide, Annex 11. Computerised Systems Ø CGMP ü 21 CFR Part 11, Electronic Records; Electronic Signatures Ø Japan GMP ü MHLW, Guideline on Management of Computerized Systems for Marketing Authorization Holders and Manufacturers of Drugs and Quasi-drugs Ø ISPE ü GAMP 5, A Risk-Based Approach to Compliant Gx. P Computerized Systems Ø PIC/S ü Annex 11, Computerised Systems ü PI 011 -3, Good Practices for Computerised Systems in Regulated Gx. P Environments Ø ICH ü Q 7 GMP Guide for API, 12. Validation

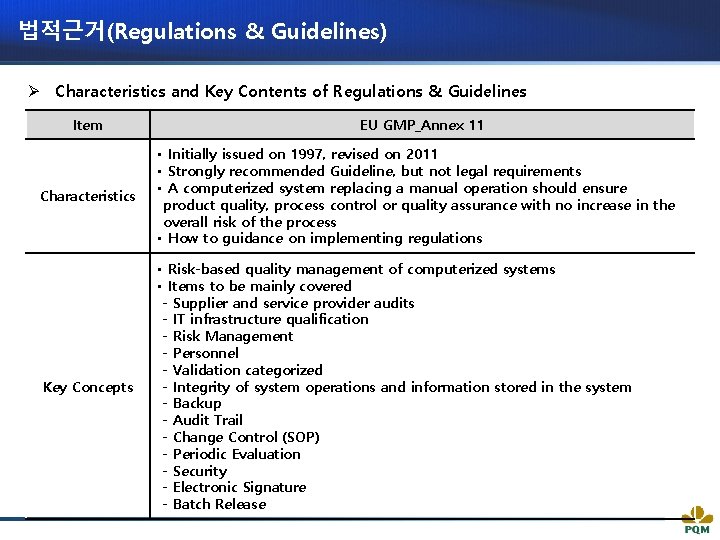
법적근거(Regulations & Guidelines) Ø Characteristics and Key Contents of Regulations & Guidelines Item EU GMP_Annex 11 Characteristics • Initially issued on 1997, revised on 2011 • Strongly recommended Guideline, but not legal requirements • A computerized system replacing a manual operation should ensure product quality, process control or quality assurance with no increase in the overall risk of the process • How to guidance on implementing regulations Key Concepts • Risk-based quality management of computerized systems • Items to be mainly covered - Supplier and service provider audits - IT infrastructure qualification - Risk Management - Personnel - Validation categorized - Integrity of system operations and information stored in the system - Backup - Audit Trail - Change Control (SOP) - Periodic Evaluation - Security - Electronic Signature - Batch Release

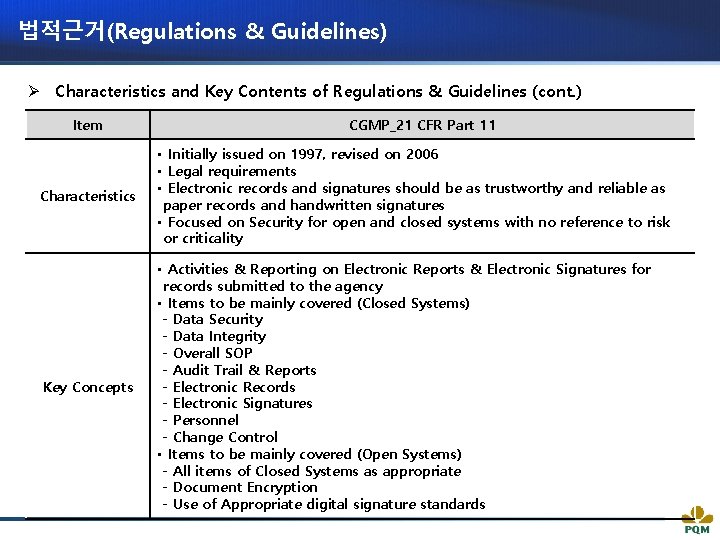
법적근거(Regulations & Guidelines) Ø Characteristics and Key Contents of Regulations & Guidelines (cont. ) Item CGMP_21 CFR Part 11 Characteristics • Initially issued on 1997, revised on 2006 • Legal requirements • Electronic records and signatures should be as trustworthy and reliable as paper records and handwritten signatures • Focused on Security for open and closed systems with no reference to risk or criticality Key Concepts • Activities & Reporting on Electronic Reports & Electronic Signatures for records submitted to the agency • Items to be mainly covered (Closed Systems) - Data Security - Data Integrity - Overall SOP - Audit Trail & Reports - Electronic Records - Electronic Signatures - Personnel - Change Control • Items to be mainly covered (Open Systems) - All items of Closed Systems as appropriate - Document Encryption - Use of Appropriate digital signature standards

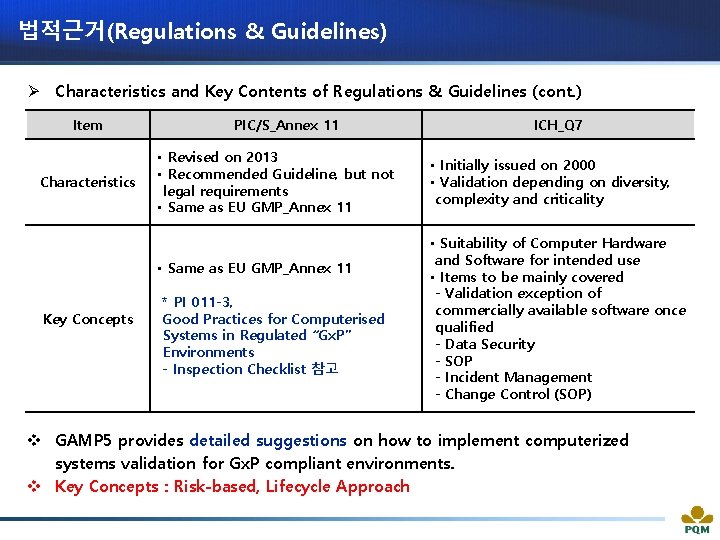
법적근거(Regulations & Guidelines) Ø Characteristics and Key Contents of Regulations & Guidelines (cont. ) Item Characteristics PIC/S_Annex 11 • Revised on 2013 • Recommended Guideline, but not legal requirements • Same as EU GMP_Annex 11 Key Concepts * PI 011 -3, Good Practices for Computerised Systems in Regulated “Gx. P” Environments - Inspection Checklist 참고 ICH_Q 7 • Initially issued on 2000 • Validation depending on diversity, complexity and criticality • Suitability of Computer Hardware and Software for intended use • Items to be mainly covered - Validation exception of commercially available software once qualified - Data Security - SOP - Incident Management - Change Control (SOP) v GAMP 5 provides detailed suggestions on how to implement computerized systems validation for Gx. P compliant environments. v Key Concepts : Risk-based, Lifecycle Approach

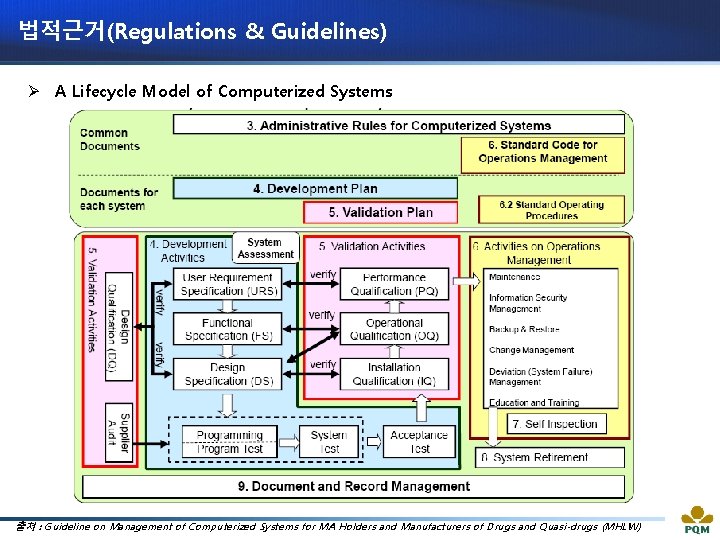
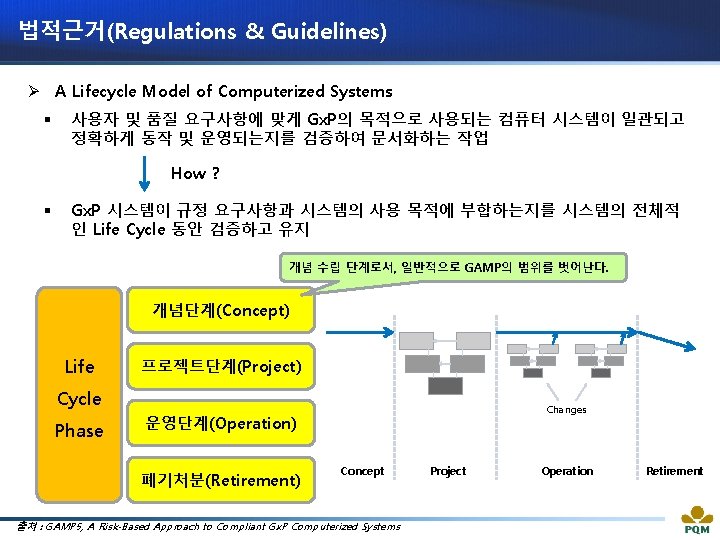
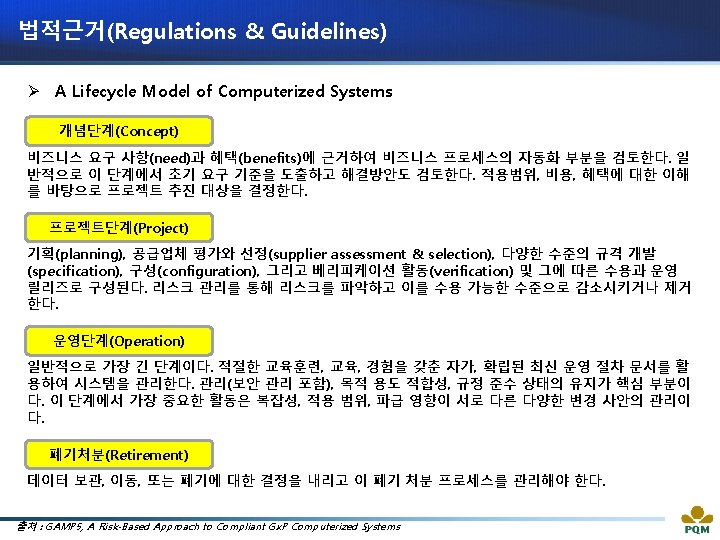
법적근거(Regulations & Guidelines) Ø A Lifecycle Model of Computerized Systems 출처 : Guideline on Management of Computerized Systems for MA Holders and Manufacturers of Drugs and Quasi-drugs (MHLW)






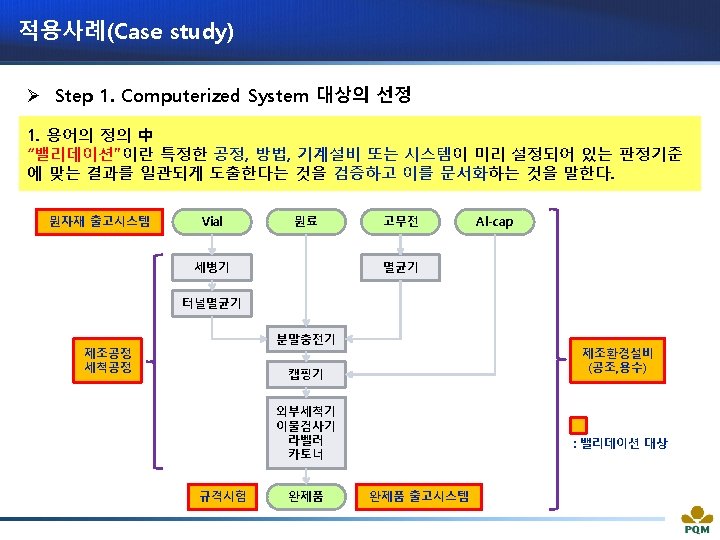
적용사례(Case study) 출처 : Guideline on Management of Computerized Systems for MA Holders and Manufacturers of Drugs and Quasi-drugs (MHLW)

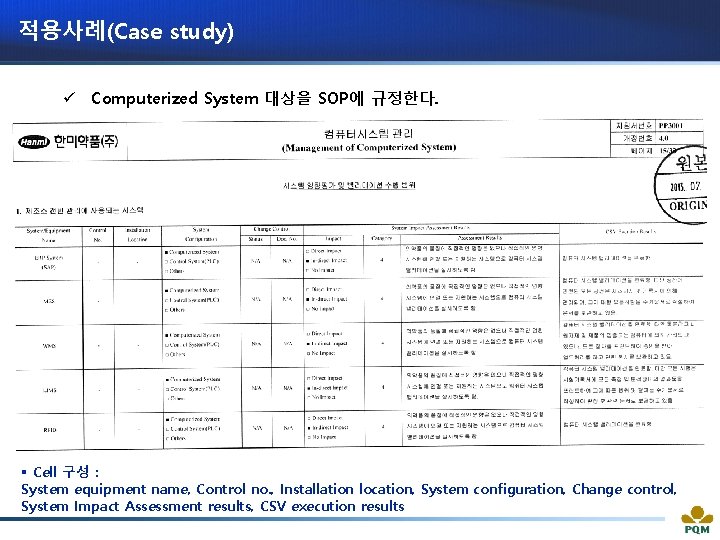
적용사례(Case study) ü Computerized System 대상을 SOP에 규정한다. § Cell 구성 : System equipment name, Control no. , Installation location, System configuration, Change control, System Impact Assessment results, CSV execution results


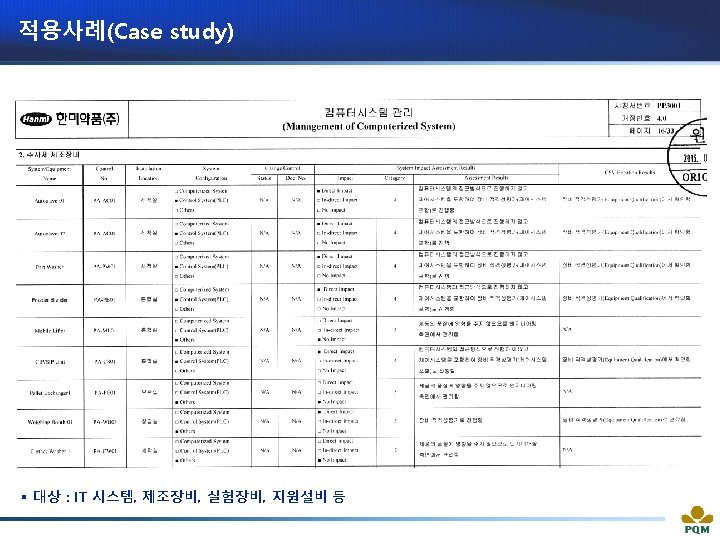
적용사례(Case study) ü System Impact Assessment 통해 CSV 범위를 설정한다.




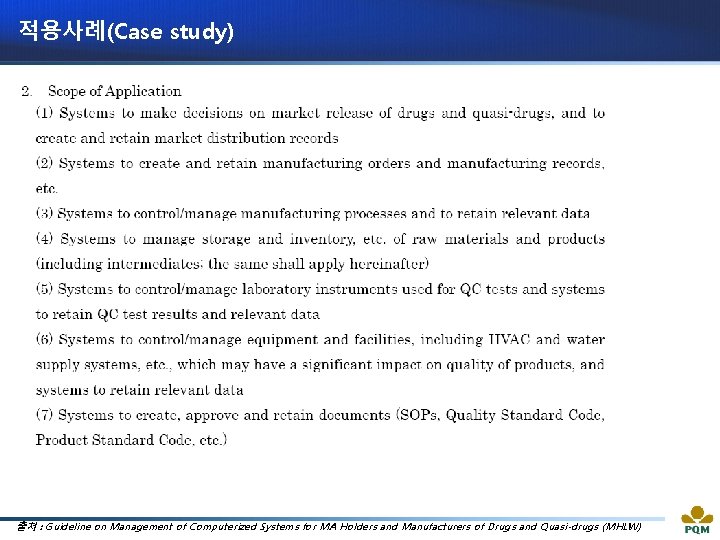
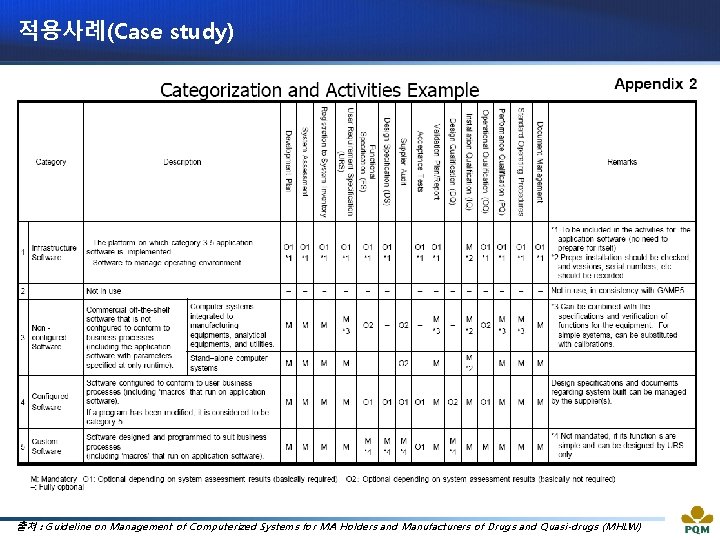
적용사례(Case study) 출처 : Guideline on Management of Computerized Systems for MA Holders and Manufacturers of Drugs and Quasi-drugs (MHLW)

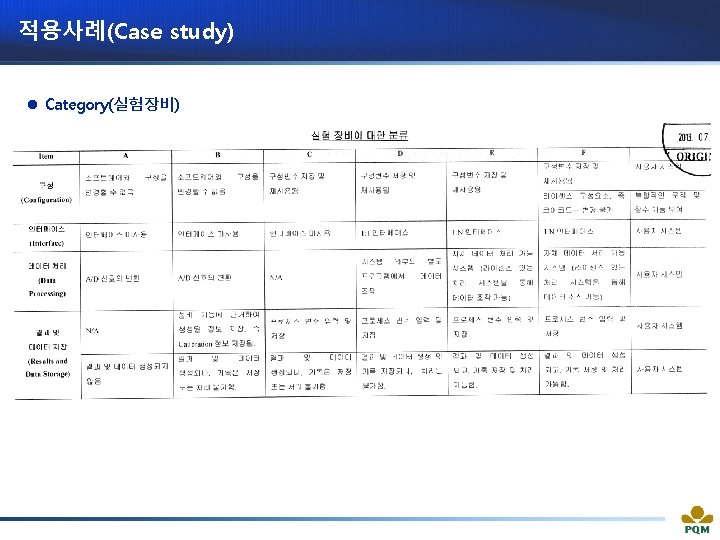
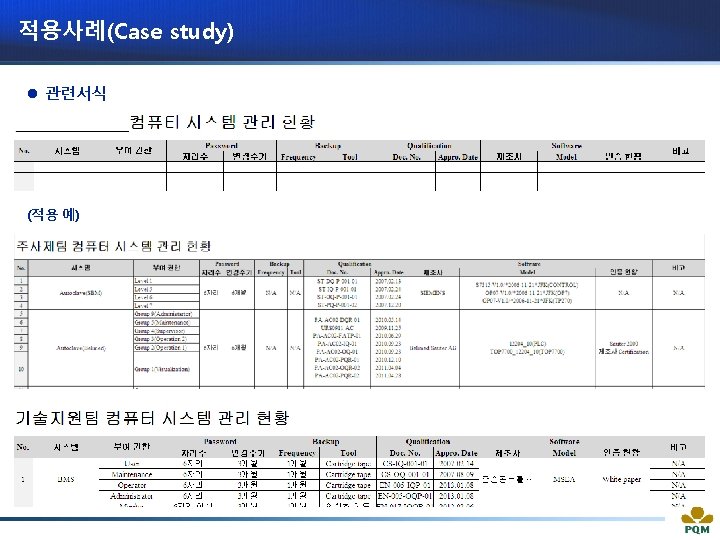
적용사례(Case study) l Category(실험장비)

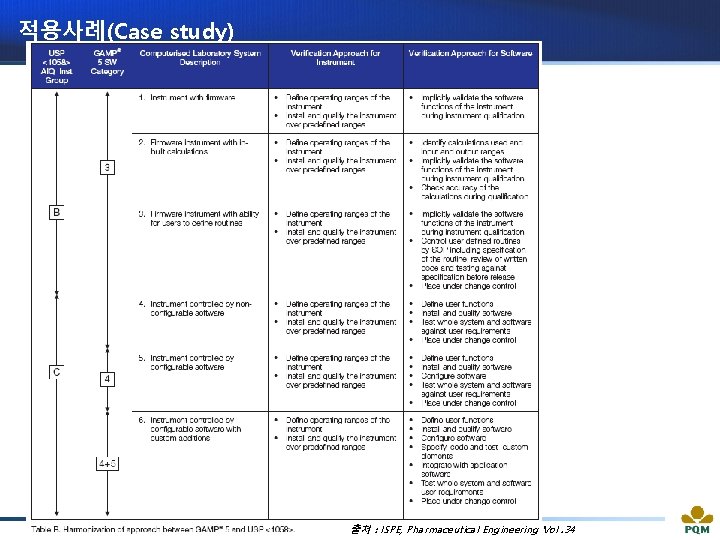
적용사례(Case study) 출처 : ISPE, Pharmaceutical Engineering Vol. 34










자동화장치 관리(CSV) Thank you Global Creative Challenge 연락처 : swdo@hanmi. co. kr 전화 : 031) 8053 - 1153 한미약품㈜ / 평택공단 세파플랜트 도승욱 팀장