Simulation Of Bioprocess ERT 3154 Introduction Stages of

Simulation Of Bioprocess ERT 315/4

Introduction
Stages of Biotech • Ancient • Classical • Modern
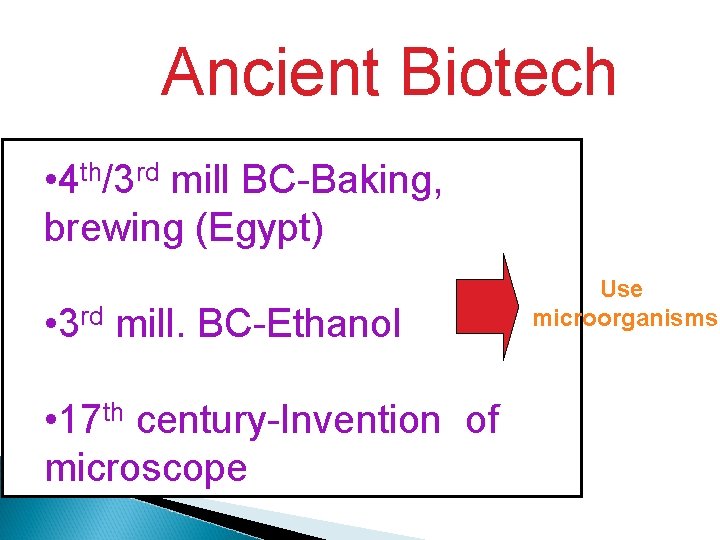
Ancient Biotech rd mill BC-Baking, with early • 4 th • /3 Begins civilization brewing (Egypt) Use • Developments in microorganisms • 3 rd mill. BC-Ethanol agriculture and food • 17 thproduction century-Invention of microscope • Few records exist
Classical Biotech • Follows ancient • Makes wide spread use of methods from ancient, especially fermentation • Methods adapted to industrial production
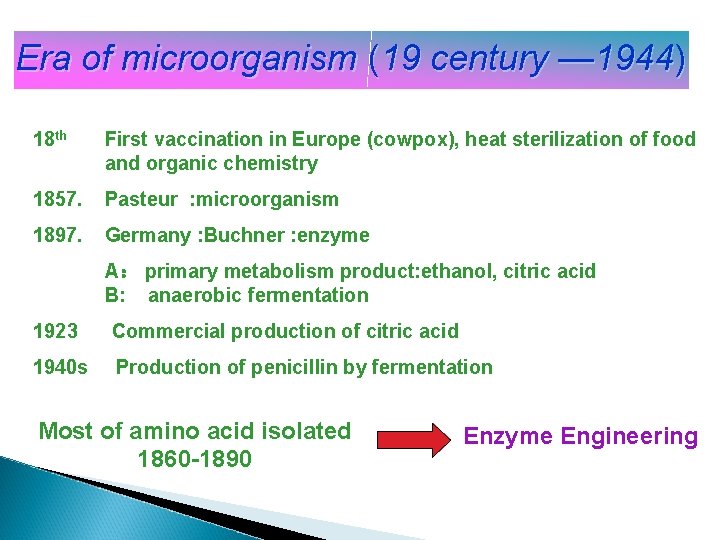
Era of microorganism (19 century — 1944) 18 th First vaccination in Europe (cowpox), heat sterilization of food and organic chemistry 1857. Pasteur : microorganism 1897. Germany : Buchner : enzyme A: primary metabolism product: ethanol, citric acid B: anaerobic fermentation 1923 Commercial production of citric acid 1940 s Production of penicillin by fermentation Most of amino acid isolated 1860 -1890 Enzyme Engineering
2. Enzyme Engineering 1953. Grubhofer and Schleith immobilization of enzyme 1969. Japan : application of immobilized enzyme in industry Amino acid production 1976 Genentech first specialist biotech company

Modern Biotech • Manipulation of genetic material within organisms • Based on genetics and the use of microscopy, biochemical methods, related sciences and technologies
3. Genetic Engineering 1974. US Boyer and Cohen recombinant DNA 1976. first biotechnology company Genentech was established 1977. Boyer h. GH 1986 First r. DNA vaccine approve 1995 First bacterial genome sequenced 2000 Human genome sequenced

Application of Biotechnology Industry Scale Biocatalyst Ø Food Downstream industry complexity Products Biotech market share Basic chemicals Fine chemicals Detergents Health care/ cosmetics Pharma conventional biopharma Very large Low MO/enzymes Very low Medium Ø Medicine MO/enzymes Large Smallmedium Low MO MO/enzymes/ mammalian cells Organic small molecules Enzymes Proteins & small molecules Food/feed Metal mining Waste treatment Ø Chemicals Medium-high Small High MO Mammalian cells, MO Very large Medium Low MO/enzymes MO MO Ø Environmental Organic small molecules Proteins Metals Purified water Low Medium Low-medium High Medium Very Low high
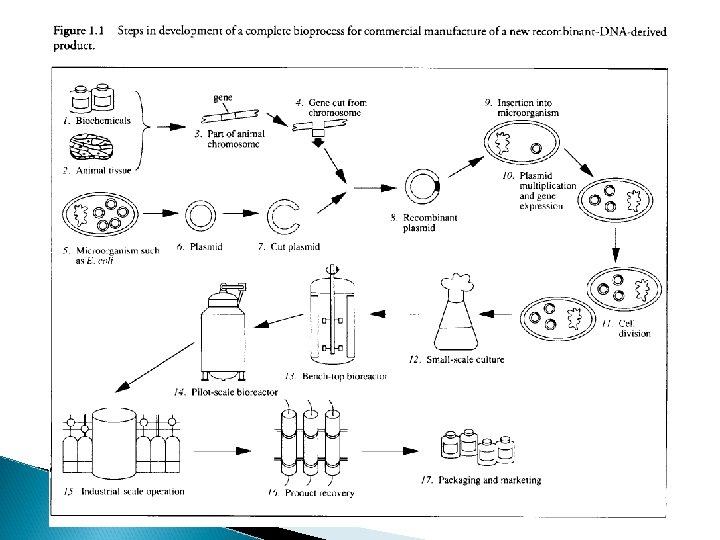

Modeling and Assessment in Process Development
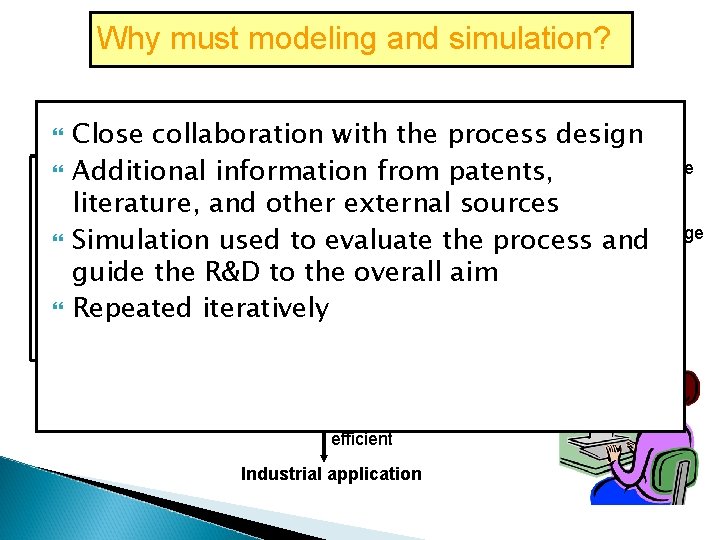
Why must modeling and simulation? Process concept Close collaboration with the process design Literature Additional information from patents, Patents Process design and Modelingsources and literature, and other external Expert development simulation knowledge Simulation used to evaluate the process and guide the R&D to the overall aim Repeated iteratively Improvement needed Sustainability assessment Ecoefficient Industrial application Not eco. Stop efficient
To gain an understanding of the actual future production To realize competitive industrial processes and decision has to be made based on the cost and potentials of a process To solve a problem that was previously overlooked rises with the development stage To give a complete picture of the expected production-scale

Development Of Bioprocess

The Biotechnical Process

Cell cultivation Enzymatic process Reactor Transgenetic Plant And Animal Fermenter Agriculture Enzymes Whole cells Extracellular Intracellular Cell harvest Product extraction Extractive technology Raw material Solid Liquid Homogenization Biomass removalsolid/liquid separation Concentration Protein refolding Product separation Viral inactivation Final formulation Crystallization Drying Final filling
Unit Operations & Unit Procedures Unit Operations: Basic step in production process e. g. sterilization, fermentation, enzymatic reaction, extraction, filtration, crystallization Unit Procedures: Set of operation that take place sequentially in a piece of equipment e. g. charging of substrate to a fermenter, addition of acid to adjust p. H, reaction, transfer of fermentation broth to another vessel

Elements of bioprocess Upstream processing Bioreactor Downstream processing

Cell cultivation Enzymatic process Reactor Transgenetic Plant And Animal Fermenter Agriculture Upstream processing Enzymes Whole cells Extracellular Intracellular Cell harvest Product extraction Extractive technology Raw material Solid Liquid Homogenization Biomass removalsolid/liquid separation Bioreactor Concentration Protein refolding Product separation Viral inactivation Downstream processing Final formulation Crystallization Drying Final filling

Upstream processing 1. Preparation and Storage of Solutions -to provide and store that are needed at some point in the process e. g. preparation of the medium for the bioreactor/ buffers in the chromatography -Liquid and solid mixture: filled in tank, mixed by agitation, stored in the tank or transferred to a separate storage tank until is needed in the process -Raw material solutions: prepared with high concentrations to keep the volume of the preparation tanks small -Carbon and nitrogen sources: prepared in separate tanks to avoid Maillard or non-enzymatic browning reactions
Upstream processing 2. Sterilization of Input Materials -to preclude contamination of the bioreactor (i) Filtration -to sterilize gaseous streams -membrane filters: Pore size- 0. 2 -0. 3µm -prefilters used for dust and other particles (ii) Heat Sterilization -heated by steam -cooling water to bring the temperature back to normal -Temperature: 121 °C (batch), 140 -45 °C (continuous), holding time: 10 -20 min -For continuous: required heat exchanger

Upstream processing 3. Inoculum preparation -to provide a sufficient amount of active cell to inoculate the production fermenter Cleaning-in-Place (CIP) -to prepare the equipment for the next cycle or batch
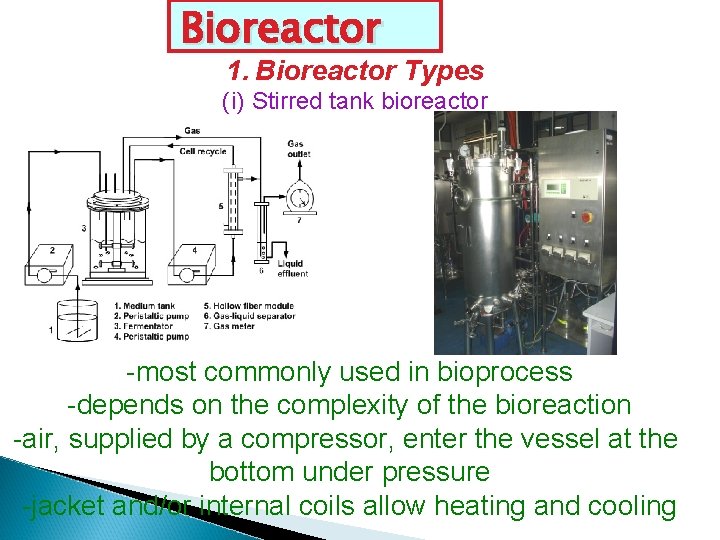
Bioreactor 1. Bioreactor Types (i) Stirred tank bioreactor -most commonly used in bioprocess -depends on the complexity of the bioreaction -air, supplied by a compressor, enter the vessel at the bottom under pressure -jacket and/or internal coils allow heating and cooling
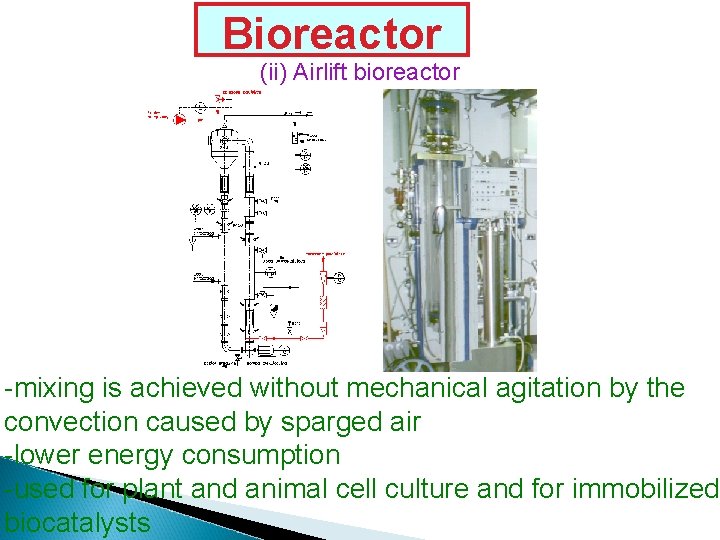
Bioreactor (ii) Airlift bioreactor -mixing is achieved without mechanical agitation by the convection caused by sparged air -lower energy consumption -used for plant and animal cell culture and for immobilized biocatalysts
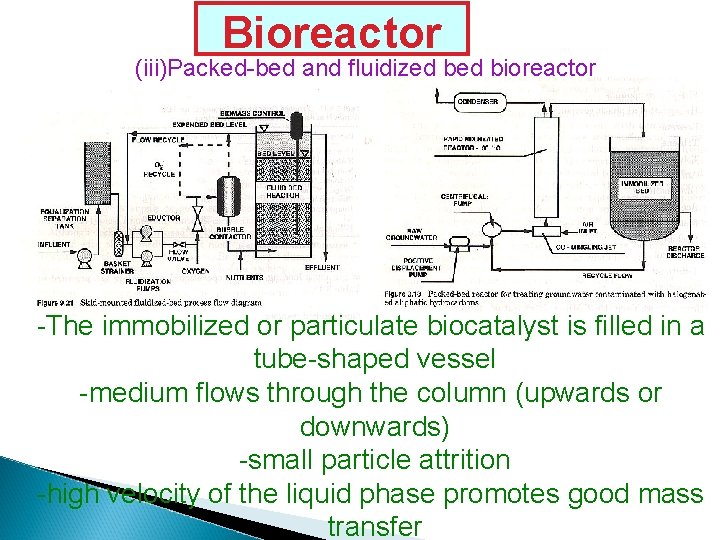
Bioreactor (iii)Packed-bed and fluidized bioreactor -The immobilized or particulate biocatalyst is filled in a tube-shaped vessel -medium flows through the column (upwards or downwards) -small particle attrition -high velocity of the liquid phase promotes good mass transfer
Bioreactor 2. Unit Procedures (i) Filling and transfer of materials in vessels -to bring materials (liquids, solids) into the bioreactor -to transfer parts or the whole reactor volume to the next operation at the end of the bioreaction -the duration should be specified -filled up to only 70 -90% to keep some headspace for foam build-up (ii) Agitation -to achieve and maintain homogeneity -to enable efficient heat transfer -energy consumption depends on the rotational speed of bioreactor, fluid density and viscosity
Bioreactor (iii) Aeration -provides oxygen to meet the aerobic demand of the cells during fermentation -specified by the gas used (Air, pure O 2, pure N 2, or air enriched with O 2 or CO 2) and the aeration rate (0. 1 and 2 vol. of gas per volume of solution per minute (vvm) (iv) Heat transfer -to change and control the temperature of the bioreactor -to keep constant while exothermic reactions take place in the fermenter -for heating, heat is transferred from a heat-transfer fluid via a heat- transfer surface to the reactor content
Bioreactor -for cooling, heat transferred from the fermentor to the cooling fluid -used steam for heating -heating rate depends on the bioreactor volume, typically at 1. 5 -3. 0 °C/min for a 10 m 3 reactor and 1 -2 °C/min for a 50 m 3 reactor -cooling agent : cooling water (20 °C), chilled water (5 °C), Freon, glycol, sodium chloride brine, calcium chloride brine (v) Foam control -to control the foam formation from the combination of agitation and aeration with the presence of foamproducing and foam-stabilizing substances

Bioreactor (vi) p. H control -to control and reach the desired p. H -the medium is buffered- adjusting and maintained the p. H by adding acid or bases (vii) Cleaning-in-place (CIP) -to clean the equipment after every batch
Downstream processing 1. Biomass removal -separate the biomass from the fermentation broth -unit operations: centrifugation, microfiltration, rotary vacuum filtration, decanting/sedimentation -depends on a number of parameter (e. g. concentration, particle size, density of biomass, scale operation etc) 2. Homogenization/Call Disruption -to break open the cells to release the product into the solution before purification -unit operation: high pressure homogenization, mechanical bead milling
Downstream processing 3. Concentration -to reduce the volume of the product stream that has to be processed -reducing equipment size and energy consumption -three methods available: (a)Partial evaporation of the solvent -solution heated up to vaporize some of the solvent, usually water (b)Filtration -semi-permeable membrane retains the product in the retentate but transfers most of the solvent through the membrane (c)Precipitation -adding a precipitation agent or by changing chemical or physical conditions
Downstream processing 4. Phase Separation (i) Centrifugation -Used for biomass removal and solid separation -based on density between solid particles and a solution between two immiscible liquids -sedimentation force is amplified by the particle or drop size in centrifugal field in the centrifuge -pretreatment is necessary to increase particle size -maximum throughput defined by the sigma factor and the settling velocity
Downstream processing (ii) Filtration -to separate particles or large molecules from a suspension or solution -semi-permeable membrane splits the components according to their size -microfiltration: (a)Pore sizes of 0. 1 -10 µm (b)Flux rate: 20 and 250 L/m 2 -ultrafiltration(a)Pore sizes of 0. 001 -0. 1 µm (b)Flux rate: 20 and 200 L/m 2
Downstream processing -dead-end filtration: (a)particles are retained as a cake through which solvent must pass (b)The pressure drop increases with solids accumulation -cross-flow filtration: (a)The feed is moved tangentially along the membrane to reduce concentration polarization or filter-cake thickness and associatedpressure drop (b) Particles are obtained as concentrated slurry -rotary vacuum filtration: Used only for large-scale filtration with large particles -diafiltration: (a) used to change the buffer solution
Downstream processing (iii) Sedimentation and decanting -sedimentation: -same as centrifugation, gravity is the driving force -needs a longer settling time and large density difference and particle size of the substances -Applied for large-scale biomass removal mostly in wastewater treatment -decanting: -for separation of liquid phases, e. g. water -the layers are formed: Solid or heavy liquid phase at the bottom, light liquid phase on top and dispersion phase in between -the parameters: density and viscosity of the two phases
Downstream processing (iv) Condensation -to liquefy the distillate in distillation (e. g. in product separation or solvent recycling) -to turn vaporized steam to liquid water after a crystallization or concentration step -use a typical shell-and-tube surface condenser-the coolant flows in the tube while condensation of the vapor occurs at the shell side -parameter: heat of vaporization, boiling point, partition coefficient of the vapor component
Downstream processing 4. Product Separation and Purification Extraction -to separate a molecule from a solution by transferring to another liquid phase -based on the different solubilities of the product and the impurities in the feed phase -used when the product concentration is comparably low or when distillation cannot be applied -differential extraction column-top: the heavy phase (aqueous solution), bottom: the light phase (organic solvent) and moves upwards. -key parameter: partition coefficient
Downstream processing (ii) Distillation -for recovery of organic solvents -based on the differences between the volatilities of substances -key parameter: Boiling point of the substances and the linear velocity of the vapor (iii) Electrodialysis -an electromotive force is used to transport ions through a semi-permeable, ion selective membrane by ion diffusion -the cations move through a cation membrane in acid stream, the anions move through an anion membrane into the supplied base stream -key parameter: membrane flux (100 -300 g/m 2 h)
Downstream processing (iv) Adsorption -to retain either the product or impurities on a solid matrix -key parameters: binding capacity and selectivity of the resin, binding yield of the target and non-target molecules, volume of the eluent (v) Chromatography -to resolve and fractionate a mixture of compounds based on differential migration -basic principles are identical to purification by adsorption -Types of column: Gel or exclusion chromatography (molecules), affinity chromatography(ligand), Ion exchange chromatography
Downstream processing 5. Viral Inactivation -to preclude contamination of the bioreactor or impurities in the product from bacteria, viruses and prions -a combination methods is necessary because none of the known methods inactivate all possible contamintants (standard purification step + additional step) -additional step : micro and ultrafiltration, heat, UV radiotion, chemical substances Protein Solubilization and Refolding -to release the intracellular material and inclusion bodies or water-insoluble pellets produced by heterologous protein in bacteria and fungi
Downstream processing 6. Final Product Processing (i) Crystallization -converted the desired product from its soluble form crystallized (solid) form -crystals are separated from the liquid solution, e. g. by filtration -initiated by a volume reduction of the solution or by reducing the solubility of the target molecules by addition of a crystallizing agents, or by changing the physical or chemical conditions -key parameter: crystallizaiton yield, crystallization heat, necessary residence time
Downstream processing (ii) Product stabilization -to avoid premature degradation or denaturation (iii) Drying -removed water or another solvent from a solid product -commonly used if the product is to be sold as powder -contact dryer: the heat is provided via the drum wall form hot water, air, or steam that flows outer side of the wall. -convection dryer: preheated drying gas is mixed with the solid and the solvent evaporates into the drying gas (iv) Filling, labeling and packing -to get the product ready for the customer or patient
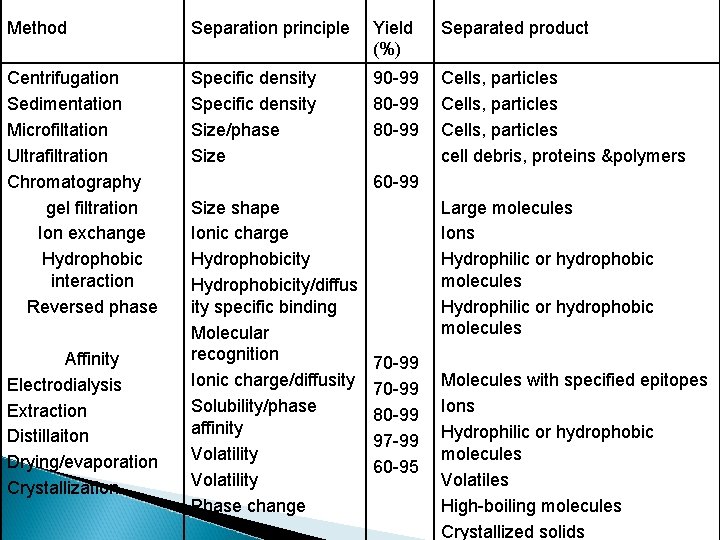
Method Separation principle Yield (%) Separated product Centrifugation Sedimentation Microfiltation Ultrafiltration Chromatography gel filtration Ion exchange Hydrophobic interaction Reversed phase Specific density Size/phase Size 90 -99 80 -99 Cells, particles cell debris, proteins &polymers Affinity Electrodialysis Extraction Distillaiton Drying/evaporation Crystallization 60 -99 Size shape Ionic charge Hydrophobicity/diffus ity specific binding Molecular recognition Ionic charge/diffusity Solubility/phase affinity Volatility Phase change Large molecules Ions Hydrophilic or hydrophobic molecules 70 -99 80 -99 97 -99 60 -95 Molecules with specified epitopes Ions Hydrophilic or hydrophobic molecules Volatiles High-boiling molecules Crystallized solids
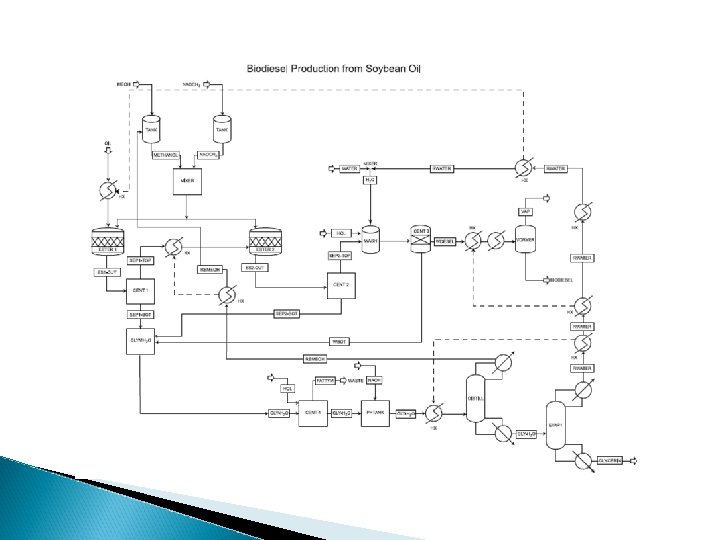

TYPES OF BIOPROCESS AND BIOPRODUCTS

Criteria to select appropriate biocatalyst q What yield, product concentration, and productivity can be reached? q What substrate can be utilized, what additional media components are required, and how does it all effect downstream processing? q What by-products are formed and how do they affect yield and downstream processing? q What are the challenges in biocatalyst preparation, storage, propagation, security, and safety? q What are the optimal reaction conditions, e. g. temperature, oxygen supply, shear sensitivity, foam formation, etc? q How well do we understand the reaction mechanisms, are they robust and genetically stable? q If the product is expressed intracellularly, how is it extracted? q How do we purify the desired product form the many impurities in the process?
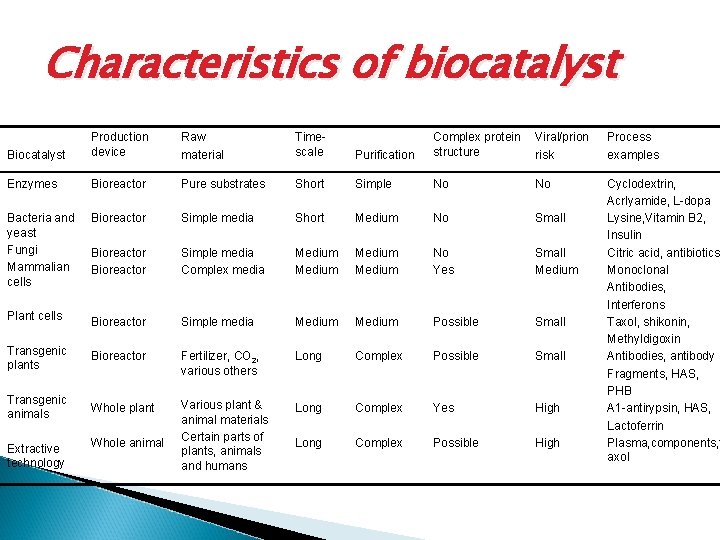
Characteristics of biocatalyst Biocatalyst Production device Raw material Timescale Purification Complex protein structure Viral/prion risk Process examples Enzymes Bioreactor Pure substrates Short Simple No No Simple media Short Medium No Small Bioreactor Simple media Complex media Medium No Yes Small Medium Plant cells Bioreactor Simple media Medium Possible Small Transgenic plants Bioreactor Fertilizer, CO 2, various others Long Complex Possible Small Transgenic animals Whole plant Various plant & animal materials Certain parts of plants, animals and humans Long Complex Yes High Long Complex Possible High Cyclodextrin, Acrlyamide, L-dopa Lysine, Vitamin B 2, Insulin Citric acid, antibiotics Monoclonal Antibodies, Interferons Taxol, shikonin, Methyldigoxin Antibodies, antibody Fragments, HAS, PHB Α 1 -antirypsin, HAS, Lactoferrin Plasma, components, t axol Bacteria and yeast Fungi Mammalian cells Bioreactor Extractive technology Whole animal
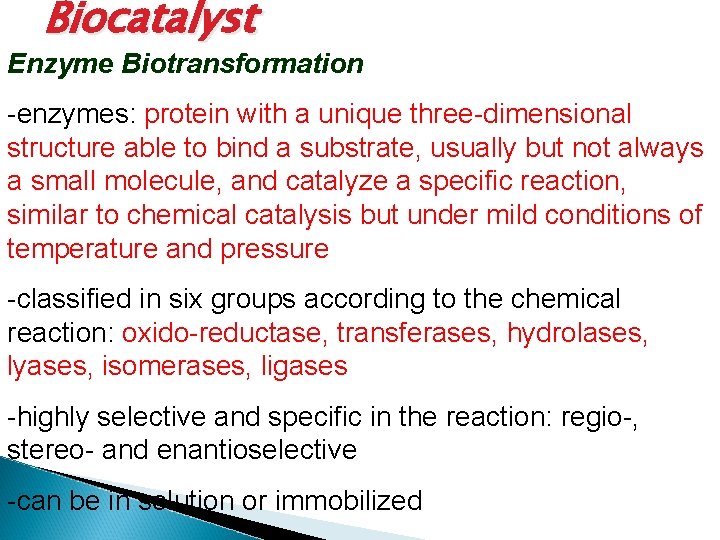
Biocatalyst Enzyme Biotransformation -enzymes: protein with a unique three-dimensional structure able to bind a substrate, usually but not always a small molecule, and catalyze a specific reaction, similar to chemical catalysis but under mild conditions of temperature and pressure -classified in six groups according to the chemical reaction: oxido-reductase, transferases, hydrolases, lyases, isomerases, ligases -highly selective and specific in the reaction: regio-, stereo- and enantioselective -can be in solution or immobilized
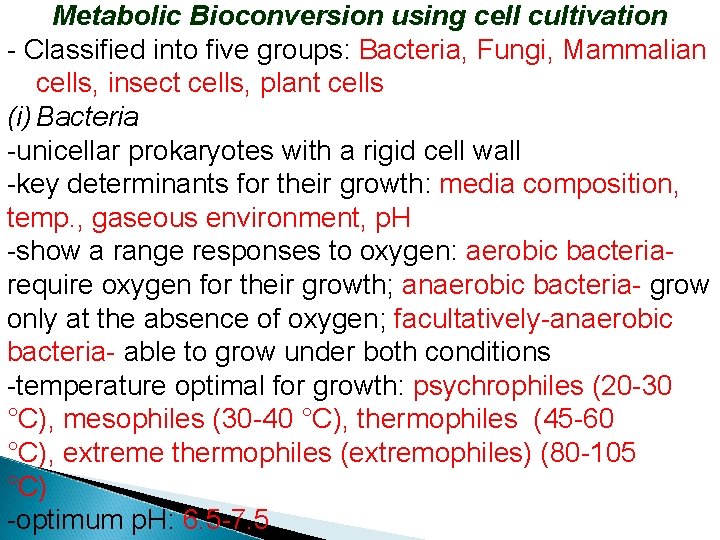
Metabolic Bioconversion using cell cultivation - Classified into five groups: Bacteria, Fungi, Mammalian cells, insect cells, plant cells (i) Bacteria -unicellar prokaryotes with a rigid cell wall -key determinants for their growth: media composition, temp. , gaseous environment, p. H -show a range responses to oxygen: aerobic bacteriarequire oxygen for their growth; anaerobic bacteria- grow only at the absence of oxygen; facultatively-anaerobic bacteria- able to grow under both conditions -temperature optimal for growth: psychrophiles (20 -30 °C), mesophiles (30 -40 °C), thermophiles (45 -60 °C), extreme thermophiles (extremophiles) (80 -105 °C) -optimum p. H: 6. 5 -7. 5

(ii) Fungi -divide into two subgroups: yeasts, molds -yeast: small, single cells that can grow as individual cells or clumps -molds: multicellular, vegetative structure call mycelium, as usually highly-branched systems of tubules (iii) Mammalian cells -produce correctly folded proteins and secrete them to the culture environment -grow quite slowly, with typical doubling times of 12 -20 h -temperature : 37 °C, p. H: 7. 3 -mammalian cell product: monoclonal antibodies, interferons, vaccines, erythropoietin
(iv) Insect cells -produce recombinant proteins less expensively and more quickly than mammalian cells and at high expression levels -typically grow at around 28 °C, and p. H 6. 2 -used for veterinary vaccines for the swine fever virus (v) Plant cells -10 to 100 times larger than microbial cells and more sensitive to shear -slow metabolism, with doubling times of 20 -100 h -cultivate as a callus or a lump of undifferentiated plant tissue growing on a solid nutrient medium or as aggregated plant cells in suspension -used to produce secondary metabolites, anticancer drug paclitexel (taxol), recombinant proteins of high value
Transgenic Plants -genetically modified plants to produce a wide variety of products -the expression can take place in the whole plant or only in a certain part as in the seeds -commonly used plants: tobacco, potato, rice, wheat -inexpensive, easy to scale-up, free of human pathogens Transgenic Animals -reduce the dependency on the seasonal and geographical conditions, post-translational modifications are more likely to mimic the native structure -usually done by injecting exogenous DNA into egg cells to produce a vital embryo that is later able to express the Desired product Extractive Technologies -comprise all processes where a product is extracted from natural material -Used in the extraction of pharmaceuticals from human or animal blood and from plant material -the products usually chemically complex non-protein materials

Bioproducts Product Classifications/Characteristics -according to size, bioproducts can be divided into: Small molecules, Large molecules and Solid particles -Small molecules i. sugars, amino acids, organic acids, vitamins ii. Molecular weight of 30 -600 Da and a radius smaller than 1 nm Iii divided into primary and secondary metabolites: a. Primary metabolites: sugar, organic alcohols, acids-produced in the primary growth face of the organism b. Secondary metabolites: formed at or near the beginning of the stationary phase, e. g. antibiotics and steroids -large molecules i. proteins, nucleic acids, polysaccharides ii. Molecular weight of 103 -106 Da -solid particle i. Whole cell like yeast, animal cell, ribosomes, viruses ii. A radius of up to several µm

-by the scale of production, bioproducts can be classified into: Bulk or commodity chemicals made at large scale, Fine chemicals and Pharmaceuticals made a smaller scale -bulk chemicals i. Produced in very large amounts ii. Simple downstream processing iii. Sold at a relatively low price -pharmaceuticals i. produced in a small amounts ii. high price iii. used expensive media and complex equipment with low productivities -fine chemicals i. Used as intermediates and have application in a variety of industries ii. Annual production, price, and required purity lie between bulk chemicals and pharmaceuticals

Product classes -describe by its function (proteins, organic acids, lipid) or application (food and feed additives, pharmaceuticals, detergents, chemical intermediates, agriculturally used products) i. organic alcohol and ketones -produced in anaerobic fermentations ii. organic acids: -used as intermediates or as food additives -major organic acids produced are citric, lactic, gluconic acid. iii. amino acids: -the building blocks of protein and are connected via peptide bonds -used as food additives, feed additives, and in pharmaceuticals iv. nucleic acids: -used as therapeutics, e. g. DNA vaccines, gene therapy v. antibiotics: -frequent use in human and animal health -produced on fungal fermentation

vi. vitamins: -produced in bioprocesses, e. g. vitamin A, C, E, and the B vitamins vii. biodegradable biopolymers: -plastics derived from renewable material -common biopolymer are polyhydroxyalkanoates (PHA) viii. dextran and xanthan: -industrially produced microbial polysaccharides -used as thickening, gelatinizing, suspending agents ix. carotenoids: -natural pigments (yellow or red color) -produced by microorganisms x. pesticides xi. lipids: -including fats, oils, waxes, phospholipids, steroids -commercially produced lipid: prostaglandins, leukotrienes, xii. proteins
Raw materials -Water: -dominant raw material -other component of the reaction medium: macronutrients and micronutrients -macronutrient: -needed in concentrations larger than 10 -4 M -including carbon–energy source, oxygen, nitrogen, phosphate, sulfur, and some minerals such as magnesium and potassium ions -carbon-energy source: -provides the carbon for biosynthesis as well as energy derived by its oxidation -Typically used carbons sources: glucose, starch, corn syrup, molasses. soybean oil, palm oil, ethanol, methanol -50% is incorporated in the biomass, and remaining 50% is used to derived energy for biosynthesis -nitrogen: -accounts for 10 -14% of the dry cell mass -most widely used are ammonia and ammonia salts, proteins,

-oxygen and hydrogen: -20% of the cell mass (O 2), 8% (H 2) -phosphorus: 3% of cell dry weight and is provided by phosphate salts -sulfate: 0. 5% of cell mass is added as sulfate salts or with amino acids contained in complex media -magnesium and potassium ions: provided as inorganic potassium and magnesium sulfate -micronutrients: -required in low concentrations: -including iron, zinc, manganese, copper, sodium, calcium, boron -added as inorganic salts -also can be classified into defined or synthetic media and complex or natural media -defined media: -contain specific amounts of pure chemicals with a known composition -complex media: -include one or more natural materials whose chemical composition is not exactly known and which may vary with source of time

-natural media: -cheaper -cause less reproducible fermentation and more complex downstram processing -bacteria and fungi: need only a relative simple media and very low cost -mammalian cells: more complex medium is necessary, need serum as required ingredient (complex media) or not (synthetic media) -plant cell: require a carbohydrate cell source, inorganic macronutrients and micronutrients.
- Slides: 61