Simplify Titration Curves that Buffer With Visual Analysis
Simplify Titration Curves that Buffer With Visual Analysis Amy Zitzelberger Hazel Park High School Hazel Park, MI Jamie Benigna The Roeper School Birmingham, MI
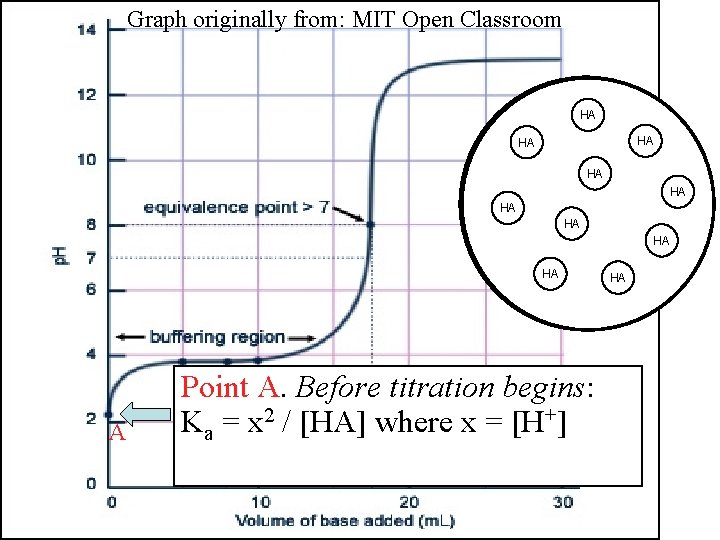
Graph originally from: MIT Open Classroom AHA HA H+ HA HA HA HA A Point A. Before titration begins: Ka = x 2 / [HA] where x = [H+] HA HA
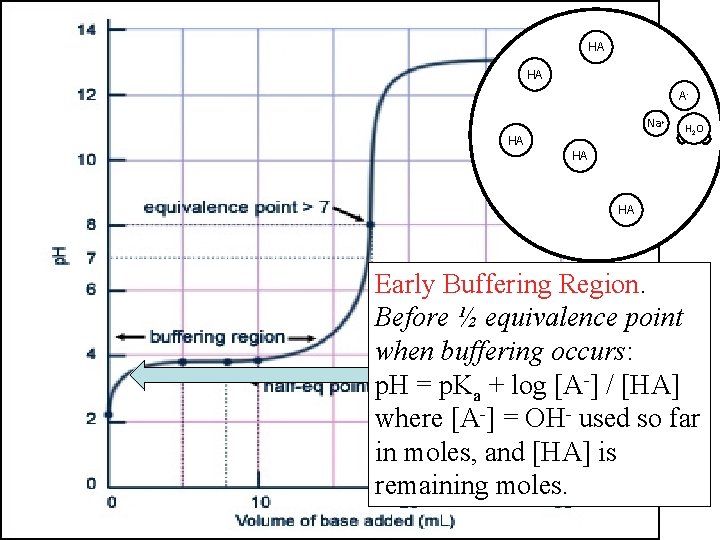
HA HA OH- A Na+ HA HA HO 2 HA HA Early Buffering Region. Before ½ equivalence point when buffering occurs: p. H = p. Ka + log [A-] / [HA] where [A-] = OH- used so far in moles, and [HA] is remaining moles.
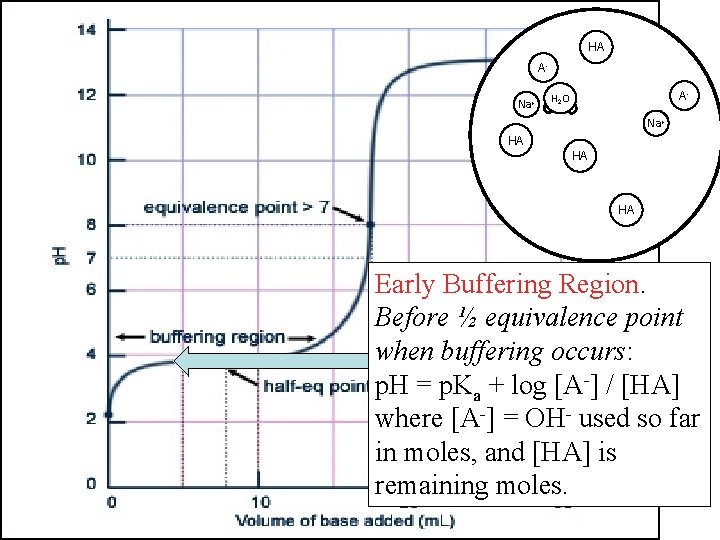
HA AHA OHNa+ A- H 2 O Na+ HA HA Early Buffering Region. Before ½ equivalence point when buffering occurs: p. H = p. Ka + log [A-] / [HA] where [A-] = OH- used so far in moles, and [HA] is remaining moles.
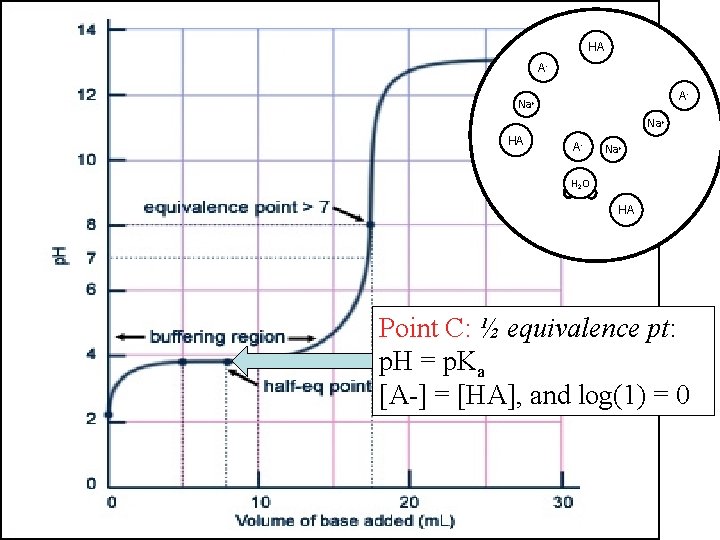
HA AHA OHA- Na+ OHHA AHA HA Na+ H 2 O HA Point C: ½ equivalence pt: p. H = p. Ka [A-] = [HA], and log(1) = 0
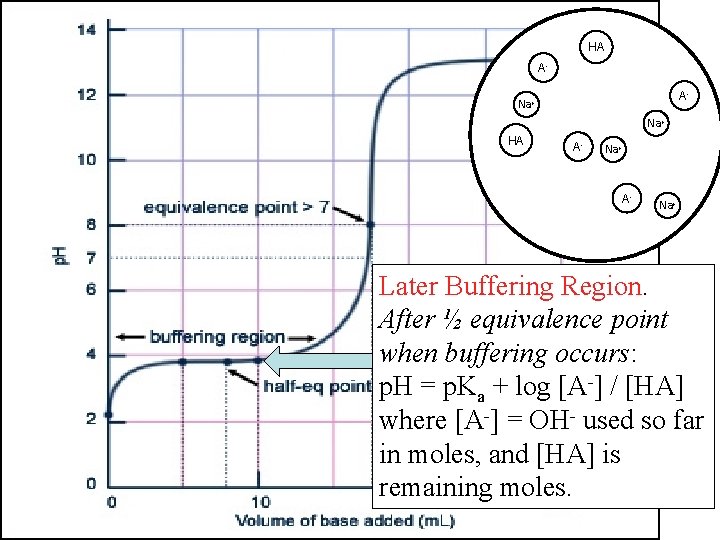
HA AHA OHA- Na+ OHHA AHA HA Na+ OHAHA Na+ Later Buffering Region. After ½ equivalence point when buffering occurs: p. H = p. Ka + log [A-] / [HA] where [A-] = OH- used so far in moles, and [HA] is remaining moles.
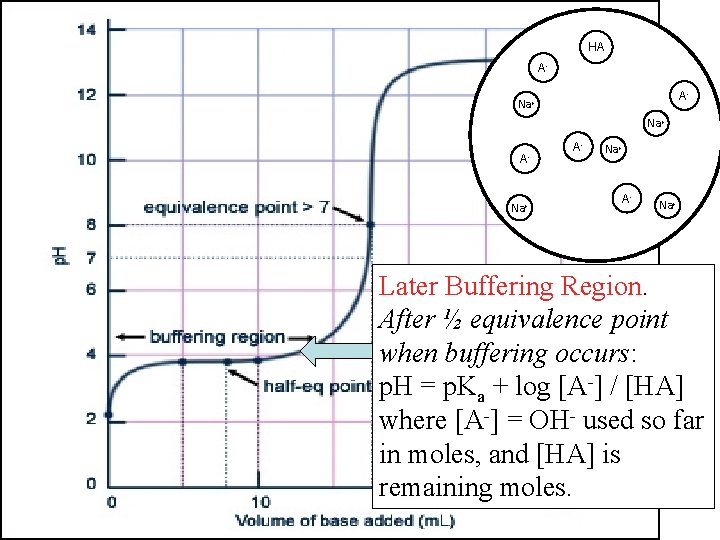
HA AHA OHA- Na+ OHHA A- OH- AHA HA Na+ OH- Na+ AHA Na+ Later Buffering Region. After ½ equivalence point when buffering occurs: p. H = p. Ka + log [A-] / [HA] where [A-] = OH- used so far in moles, and [HA] is remaining moles.
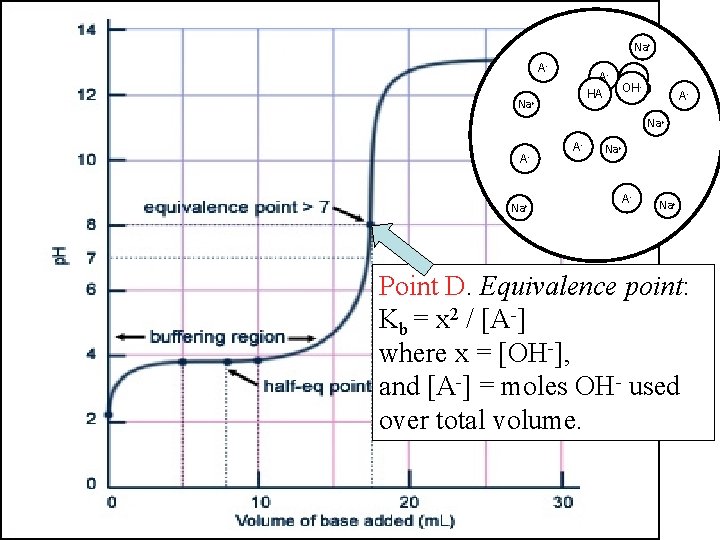
Na+ HA AHA OH- OH A-HA Na+ H 2 O OHNa+ OHHA A- OH- AHA Na+ OH- Na+ AHA Na+ Point D. Equivalence point: Kb = x 2 / [A-] where x = [OH-], and [A-] = moles OH- used over total volume.
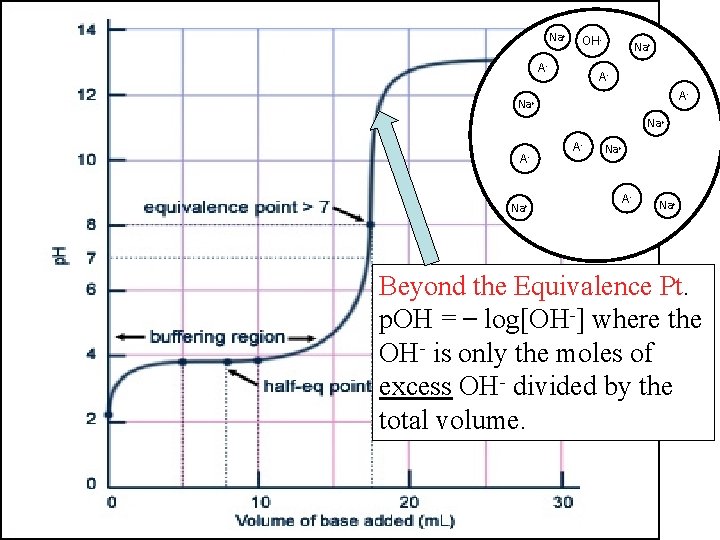
Na+ OHHA Na+ AHA OH- OH A-A- Na+ OHHA A- OH- AHA HA Na+ OH- Na+ AHA Na+ Beyond the Equivalence Pt. p. OH = log[OH-] where the OH- is only the moles of excess OH- divided by the total volume.
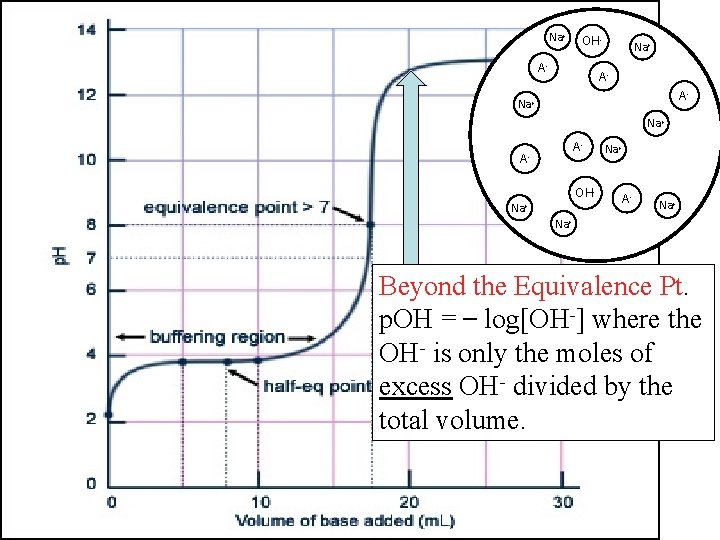
Na+ OHHA Na+ AHA OH- OH A-A- Na+ OHHA A- OH- AHA HA Na+ OH- OHNa+ AHA Na+ Beyond the Equivalence Pt. p. OH = log[OH-] where the OH- is only the moles of excess OH- divided by the total volume.
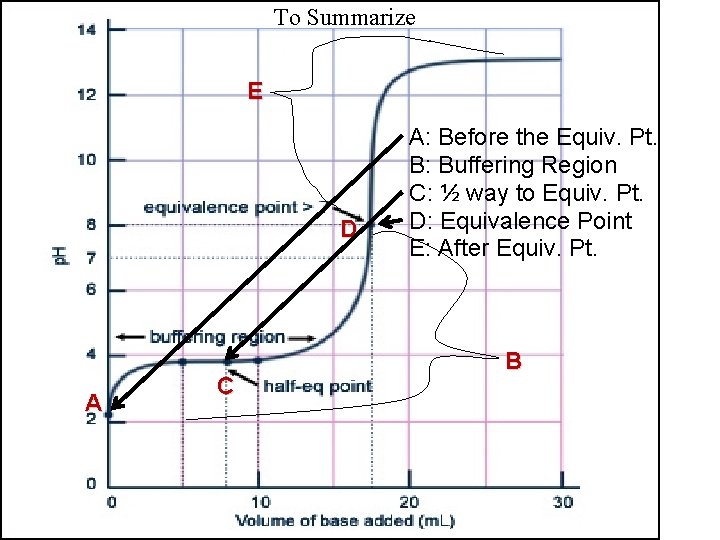
To Summarize E D A C A: Before the Equiv. Pt. B: Buffering Region C: ½ way to Equiv. Pt. D: Equivalence Point E: After Equiv. Pt. B
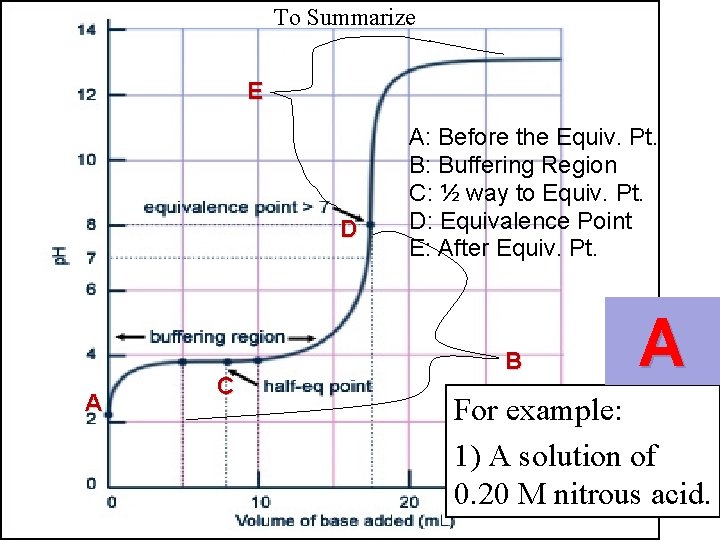
To Summarize E D A C A: Before the Equiv. Pt. B: Buffering Region C: ½ way to Equiv. Pt. D: Equivalence Point E: After Equiv. Pt. B A For example: 1) A solution of 0. 20 M nitrous acid.
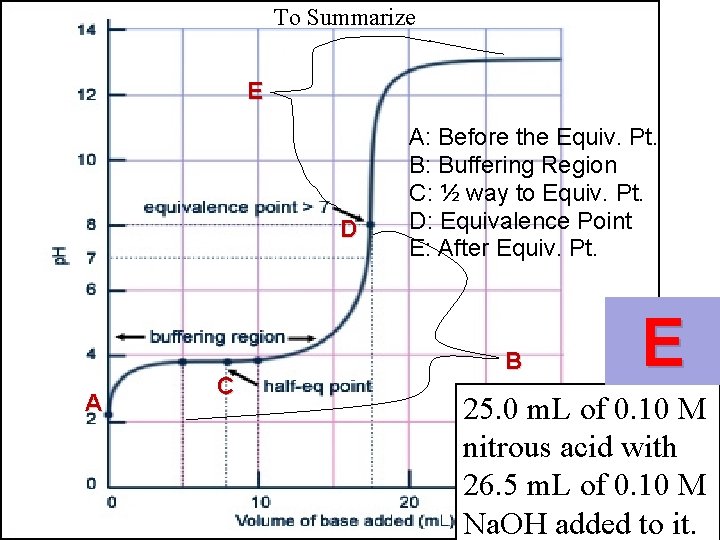
To Summarize E D A C A: Before the Equiv. Pt. B: Buffering Region C: ½ way to Equiv. Pt. D: Equivalence Point E: After Equiv. Pt. B E 25. 0 m. L of 0. 10 M nitrous acid with 26. 5 m. L of 0. 10 M Na. OH added to it.
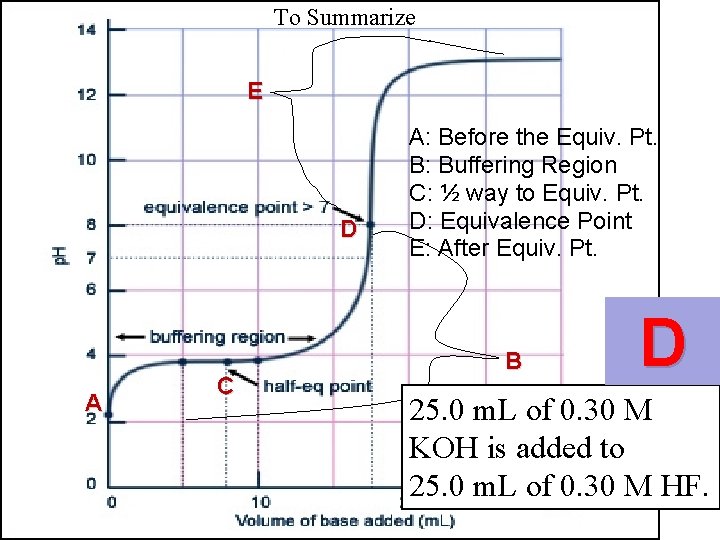
To Summarize E D A C A: Before the Equiv. Pt. B: Buffering Region C: ½ way to Equiv. Pt. D: Equivalence Point E: After Equiv. Pt. B D 25. 0 m. L of 0. 30 M KOH is added to 25. 0 m. L of 0. 30 M HF.
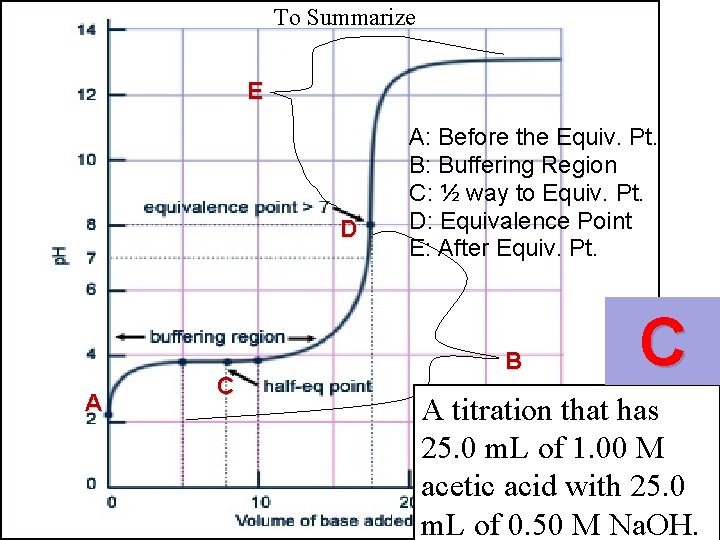
To Summarize E D A C A: Before the Equiv. Pt. B: Buffering Region C: ½ way to Equiv. Pt. D: Equivalence Point E: After Equiv. Pt. B C A titration that has 25. 0 m. L of 1. 00 M acetic acid with 25. 0 m. L of 0. 50 M Na. OH.
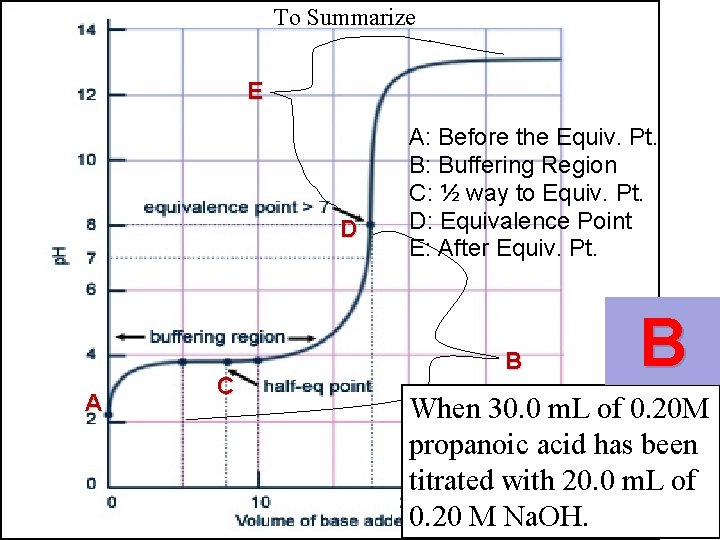
To Summarize E D A C A: Before the Equiv. Pt. B: Buffering Region C: ½ way to Equiv. Pt. D: Equivalence Point E: After Equiv. Pt. B B When 30. 0 m. L of 0. 20 M propanoic acid has been titrated with 20. 0 m. L of 0. 20 M Na. OH.
- Slides: 16