Simple Organic Chemistry Basic Structure and Nomenclature First
Simple Organic Chemistry Basic Structure and Nomenclature

First Ten Alkanes Formula Name CH 4 Methane Formula Name C 6 H 14 Hexane C 2 H 6 Ethane C 7 H 16 Heptane C 3 H 8 Propane C 8 H 18 Octane C 4 H 10 Butane C 9 H 20 Nonane C 5 H 12 Pentane C 10 H 22 Decane Alkane = Cn. H 2 n+2
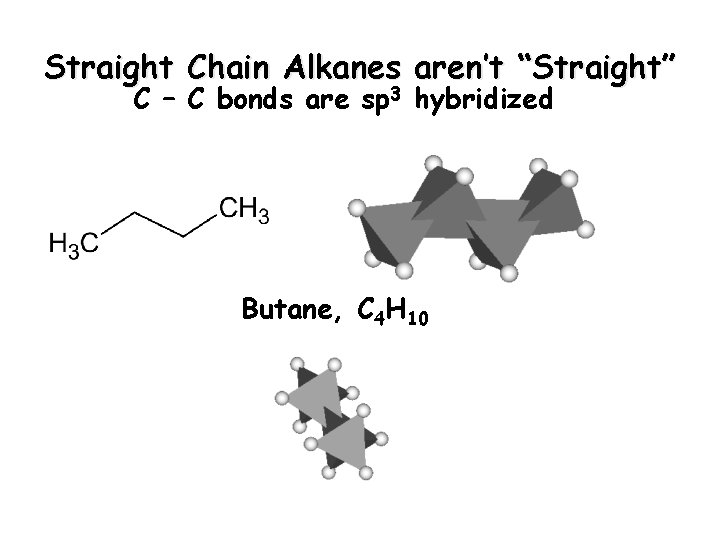
Straight Chain Alkanes aren’t “Straight” C – C bonds are sp 3 hybridized Butane, C 4 H 10
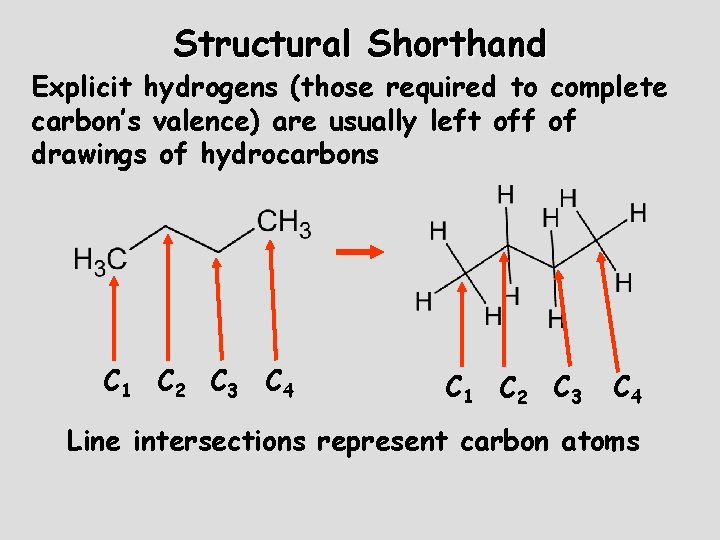
Structural Shorthand Explicit hydrogens (those required to complete carbon’s valence) are usually left off of drawings of hydrocarbons C 1 C 2 C 3 C 4 Line intersections represent carbon atoms
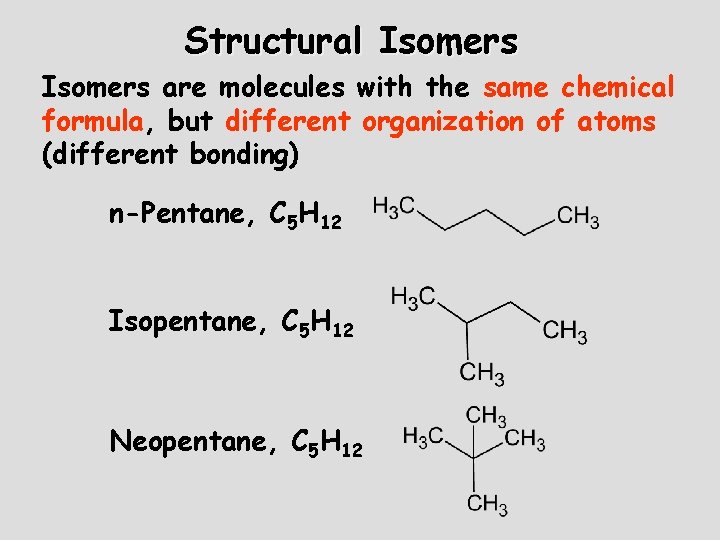
Structural Isomers are molecules with the same chemical formula, but different organization of atoms (different bonding) n-Pentane, C 5 H 12 Isopentane, C 5 H 12 Neopentane, C 5 H 12
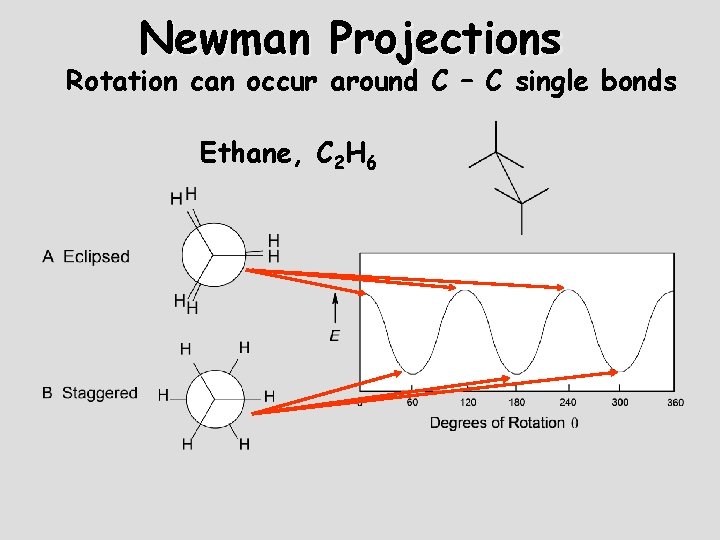
Newman Projections Rotation can occur around C – C single bonds Ethane, C 2 H 6
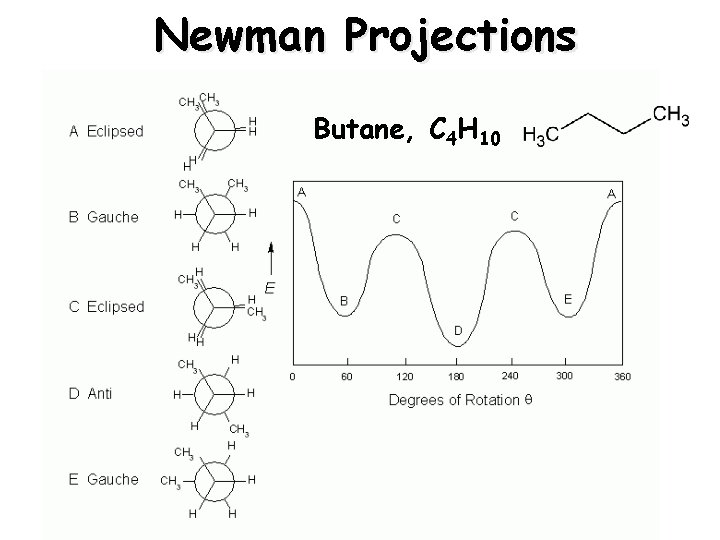
Newman Projections Butane, C 4 H 10
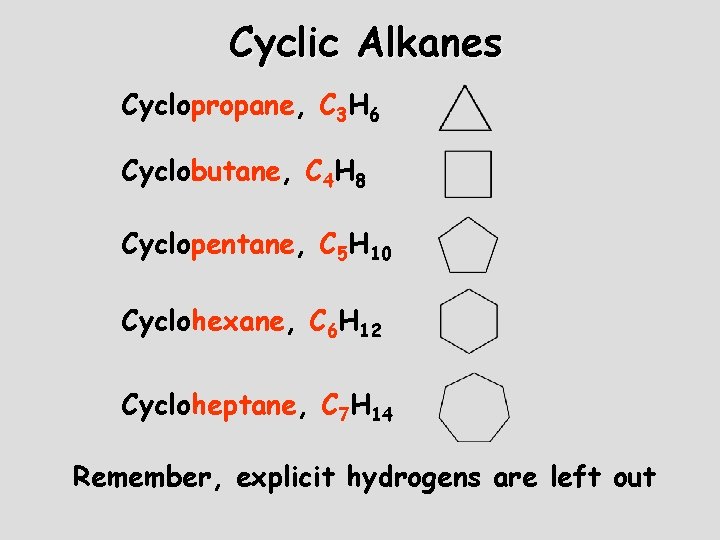
Cyclic Alkanes Cyclopropane, C 3 H 6 Cyclobutane, C 4 H 8 Cyclopentane, C 5 H 10 Cyclohexane, C 6 H 12 Cycloheptane, C 7 H 14 Remember, explicit hydrogens are left out
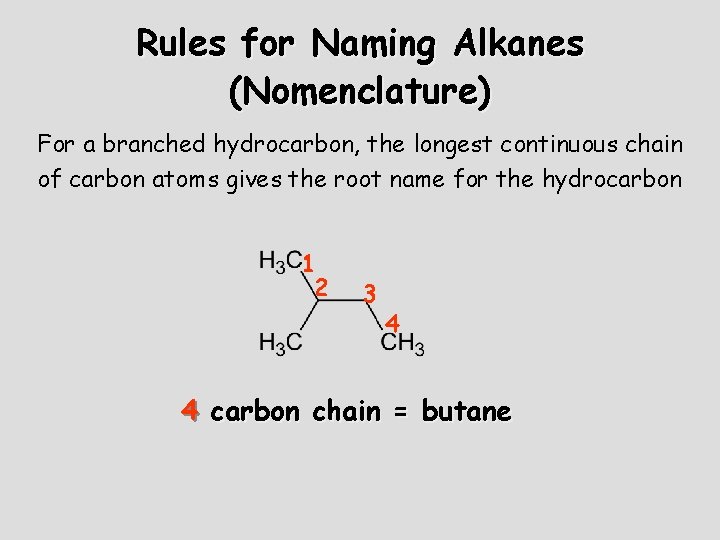
Rules for Naming Alkanes (Nomenclature) For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon 1 2 3 4 4 carbon chain = butane
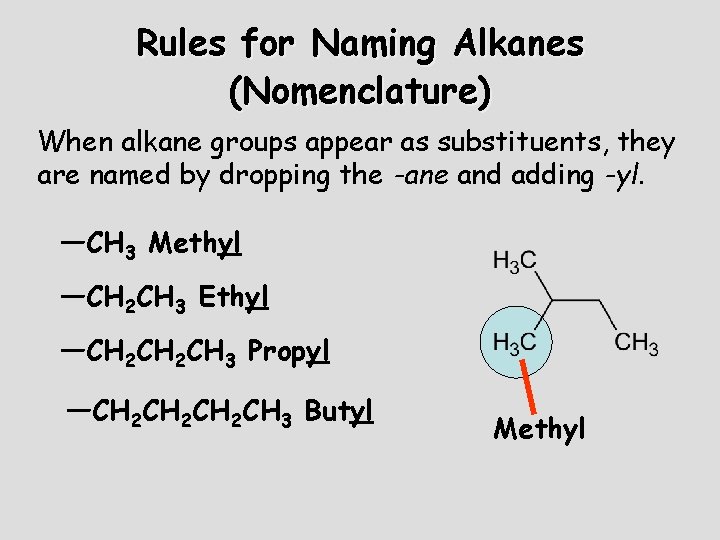
Rules for Naming Alkanes (Nomenclature) When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl. —CH 3 Methyl —CH 2 CH 3 Ethyl —CH 2 CH 3 Propyl —CH 2 CH 2 CH 3 Butyl Methyl
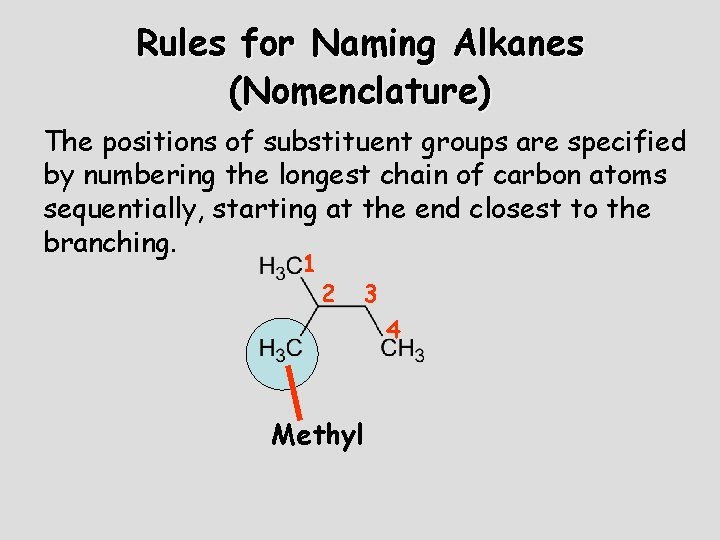
Rules for Naming Alkanes (Nomenclature) The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. 1 2 3 4 Methyl
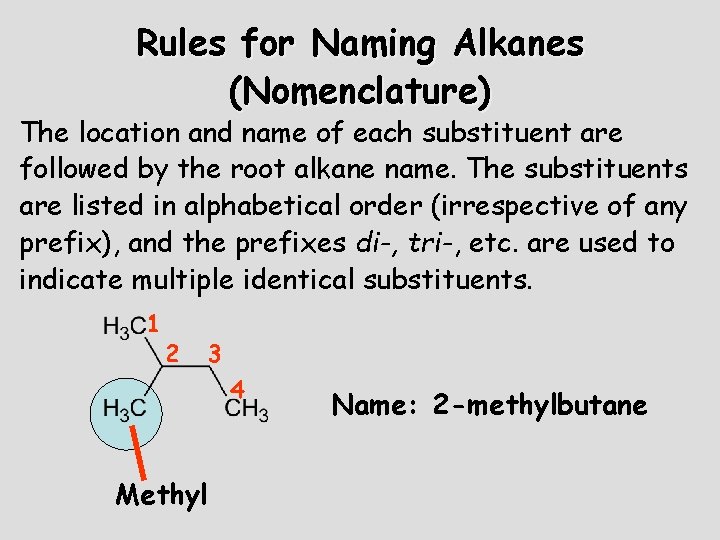
Rules for Naming Alkanes (Nomenclature) The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents. 1 2 3 4 Methyl Name: 2 -methylbutane
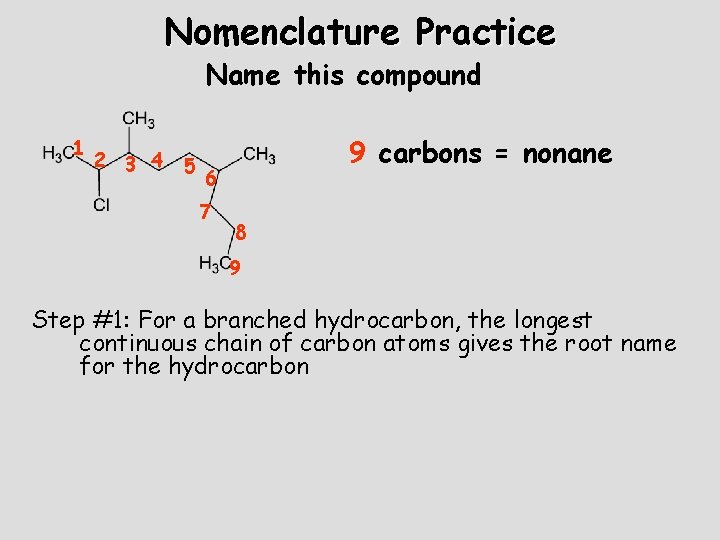
Nomenclature Practice Name this compound 1 2 3 4 5 9 carbons = nonane 6 7 8 9 Step #1: For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon
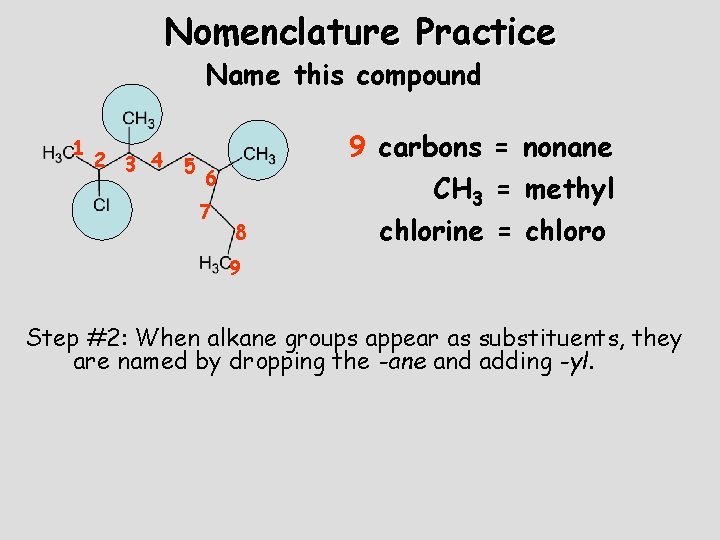
Nomenclature Practice Name this compound 1 2 3 4 5 6 7 8 9 carbons = nonane CH 3 = methyl chlorine = chloro 9 Step #2: When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl.
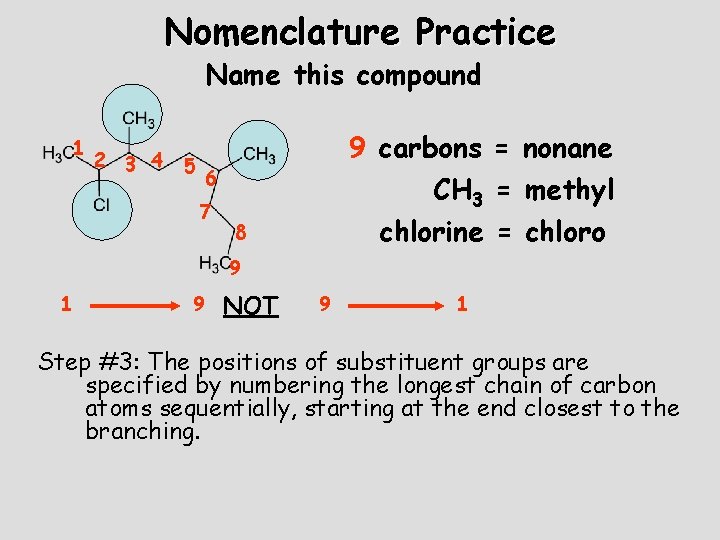
Nomenclature Practice Name this compound 1 2 3 4 5 9 carbons = nonane 6 7 CH 3 = methyl chlorine = chloro 8 9 1 9 NOT 9 1 Step #3: The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching.
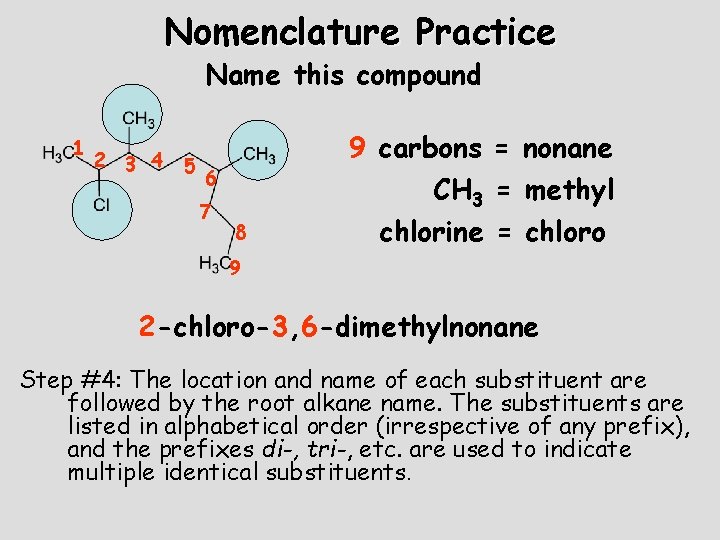
Nomenclature Practice Name this compound 1 2 3 4 5 9 carbons = nonane 6 7 8 CH 3 = methyl chlorine = chloro 9 2 -chloro-3, 6 -dimethylnonane Step #4: The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents.
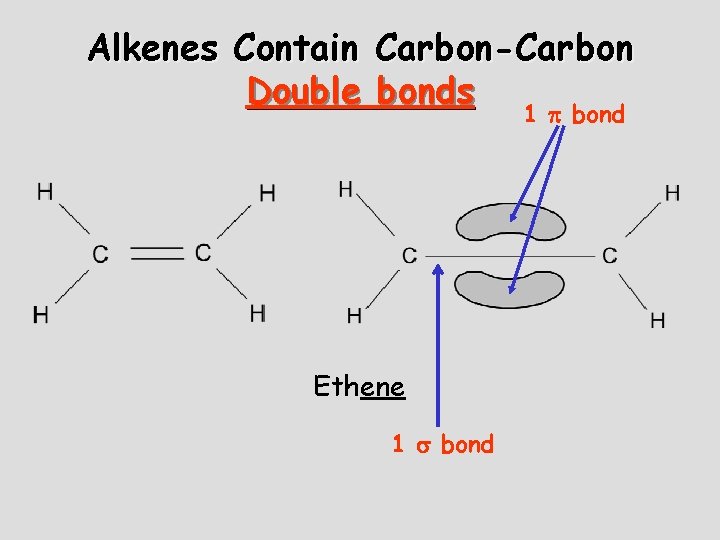
Alkenes Contain Carbon-Carbon Double bonds 1 bond Ethene 1 bond
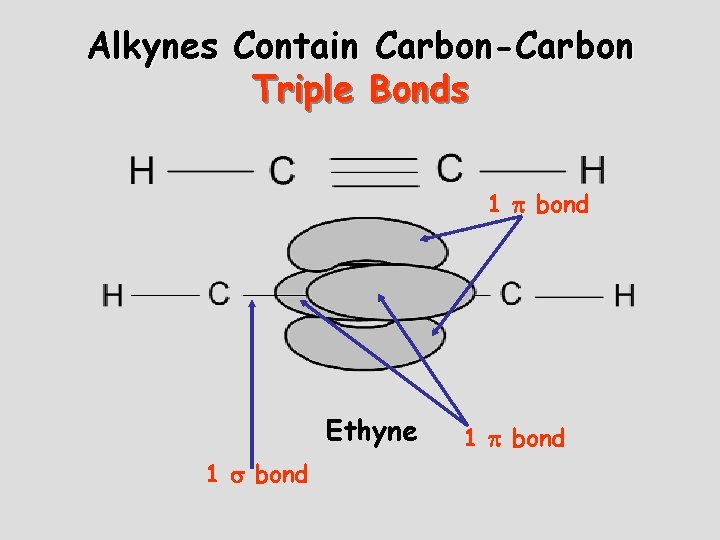
Alkynes Contain Carbon-Carbon Triple Bonds 1 bond Ethyne 1 bond
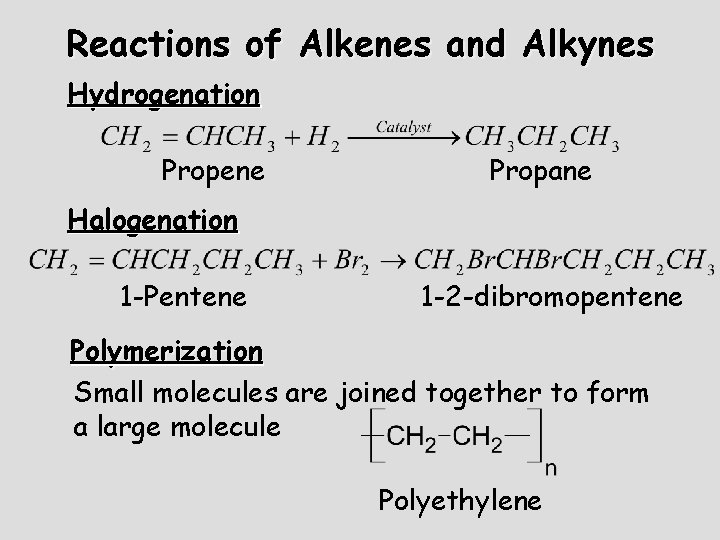
Reactions of Alkenes and Alkynes Hydrogenation Propene Propane Halogenation 1 -Pentene 1 -2 -dibromopentene Polymerization Small molecules are joined together to form a large molecule Polyethylene
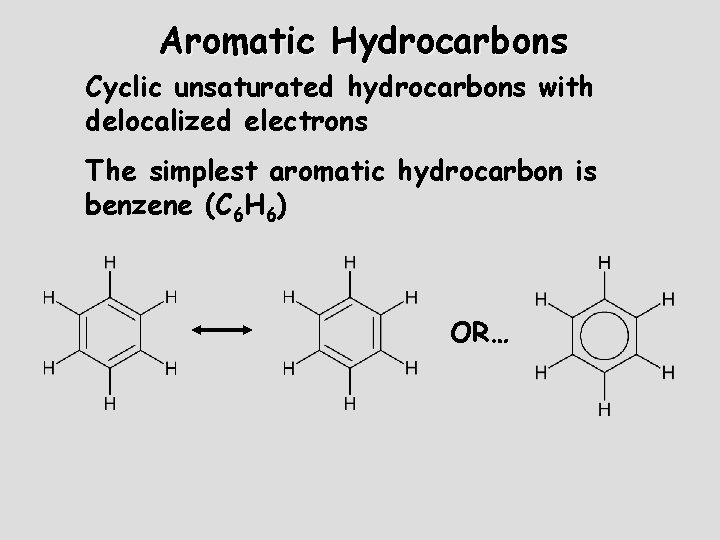
Aromatic Hydrocarbons Cyclic unsaturated hydrocarbons with delocalized electrons The simplest aromatic hydrocarbon is benzene (C 6 H 6) OR…
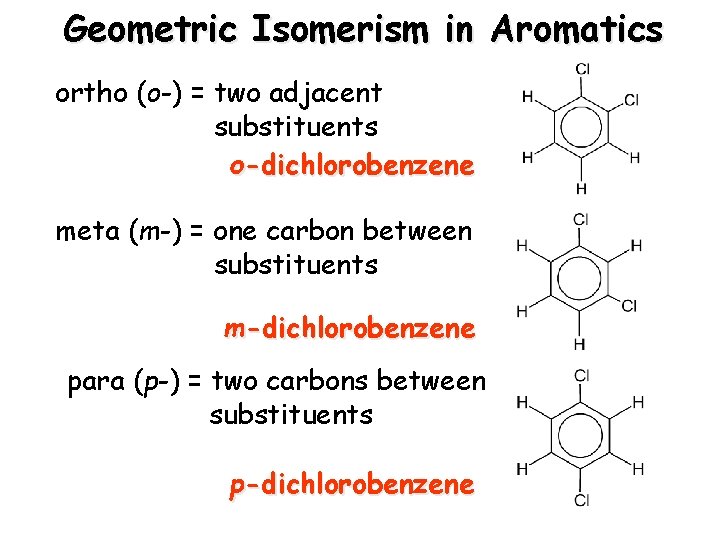
Geometric Isomerism in Aromatics ortho (o-) = two adjacent substituents o-dichlorobenzene meta (m-) = one carbon between substituents m-dichlorobenzene para (p-) = two carbons between substituents p-dichlorobenzene
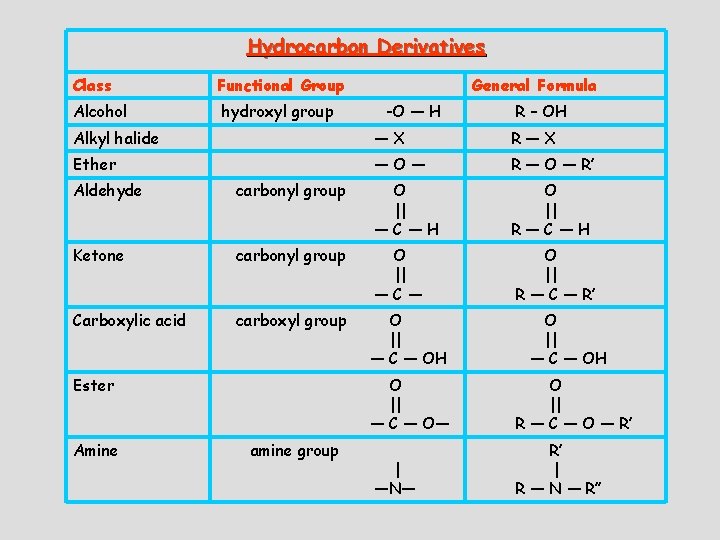
Hydrocarbon Derivatives Class Functional Group Alcohol hydroxyl group General Formula -O — H R – OH Alkyl halide —X R—X Ether —O— R — O — R’ Aldehyde carbonyl group O || —C—H O || R—C—H Ketone carbonyl group O || —C— O || R — C — R’ Carboxylic acid carboxyl group O || — C — OH Ester Amine amine group O || — C — OH O || — C — O— O || R — C — O — R’ | —N— R’ | R — N — R’’
- Slides: 22