Silica Surface Modification Reactions Kinetics Mechanisms and Surface










- Slides: 42

Silica Surface Modification Reactions: Kinetics, Mechanisms, and Surface Structures Jonathan Blitz Department of Chemistry


Applications of Surface Modified Silicas • • • Composites Adhesives/Sealants Paints/Coatings Chromatographic stationary phases Catalyst supports/Catalysts Adsorbents

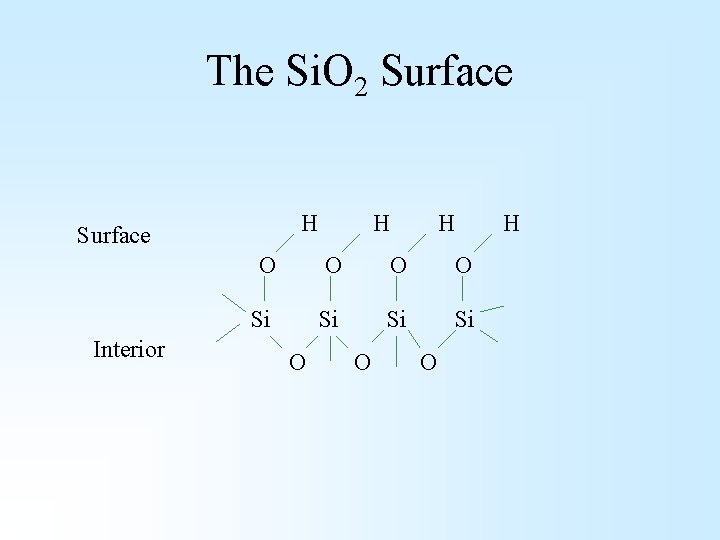
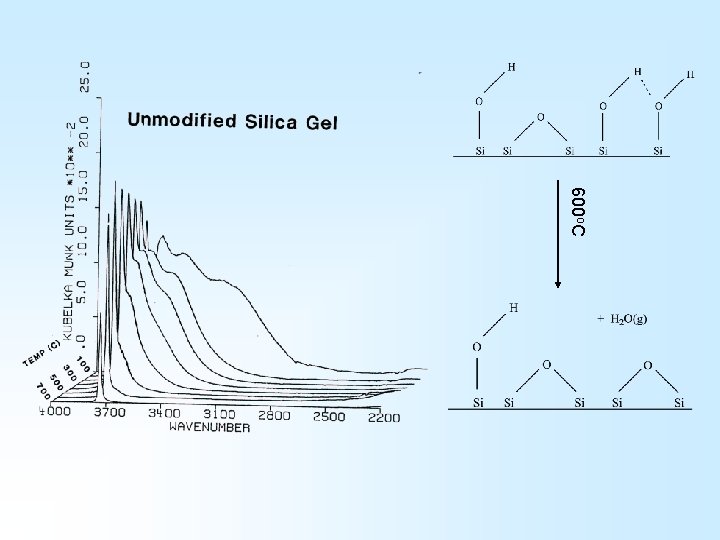
The Si. O 2 Surface H Surface Interior H H O O Si Si O O O H

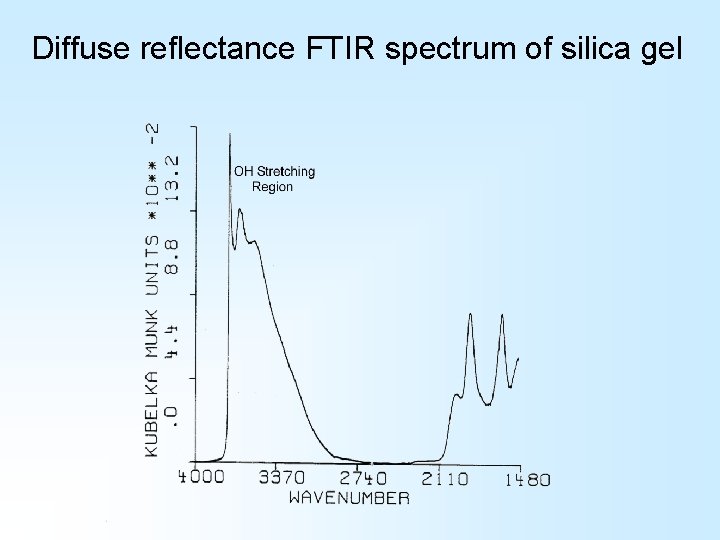
Diffuse reflectance FTIR spectrum of silica gel

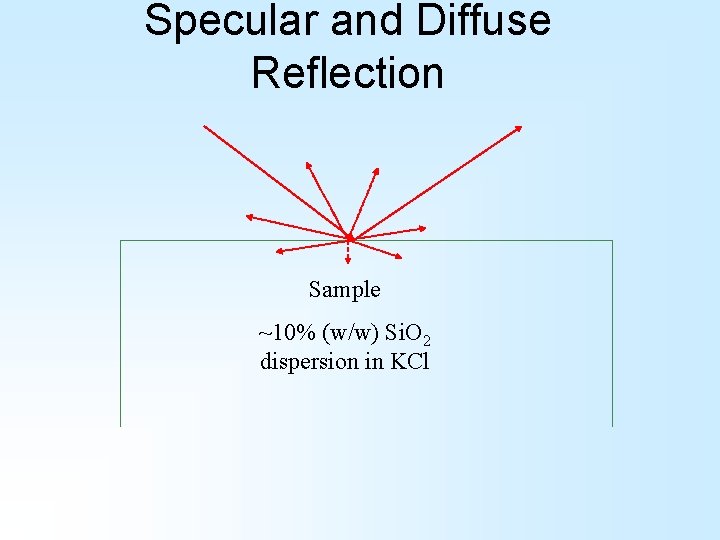
Specular and Diffuse Reflection Sample ~10% (w/w) Si. O 2 dispersion in KCl

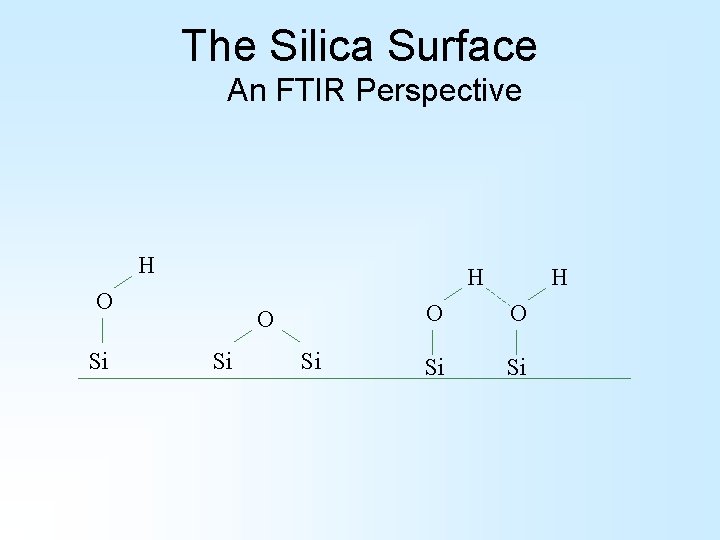
The Silica Surface An FTIR Perspective H H O Si Si H O O Si Si

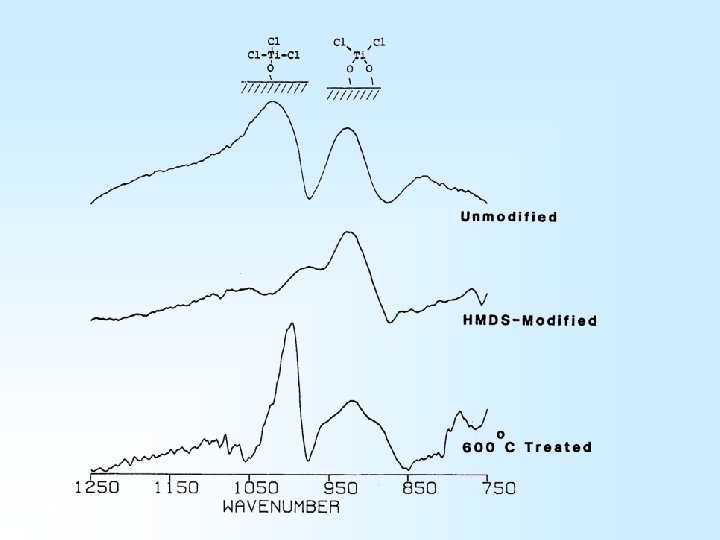
HMDS (CH 3)3 Si. NHSi(CH 3)3

600 o. C


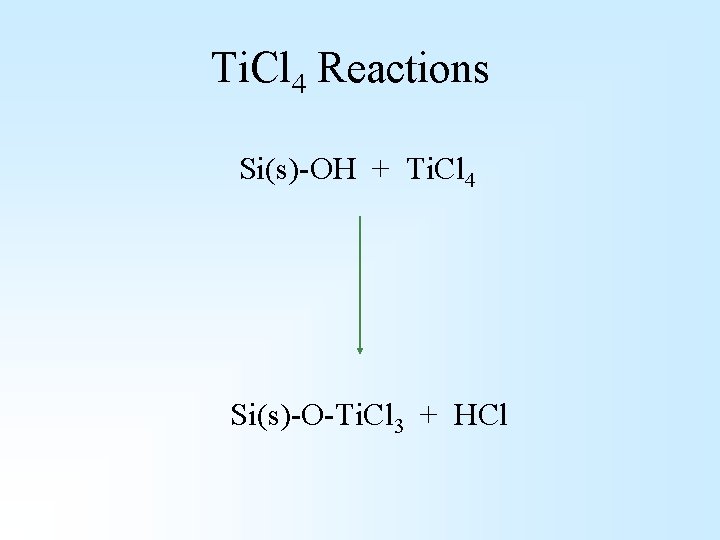
Ti. Cl 4 Reactions Si(s)-OH + Ti. Cl 4 Si(s)-O-Ti. Cl 3 + HCl



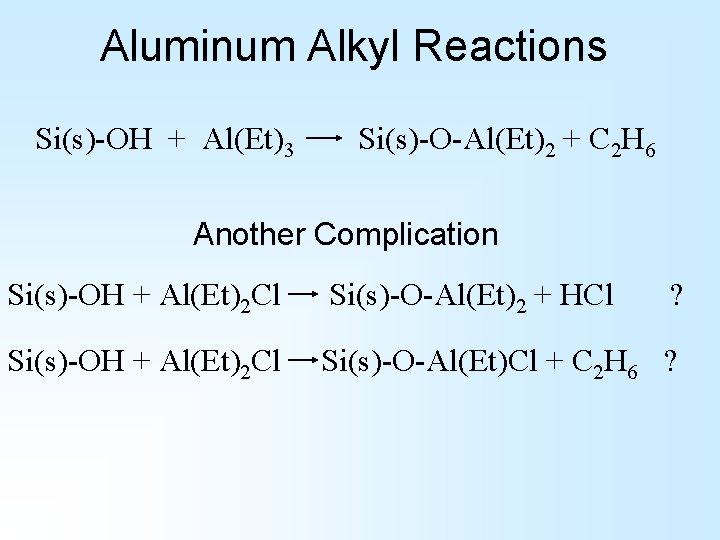
Aluminum Alkyl Reactions Si(s)-OH + Al(Et)3 Si(s)-O-Al(Et)2 + C 2 H 6 Another Complication Si(s)-OH + Al(Et)2 Cl Si(s)-O-Al(Et)2 + HCl ? Si(s)-OH + Al(Et)2 Cl Si(s)-O-Al(Et)Cl + C 2 H 6 ?

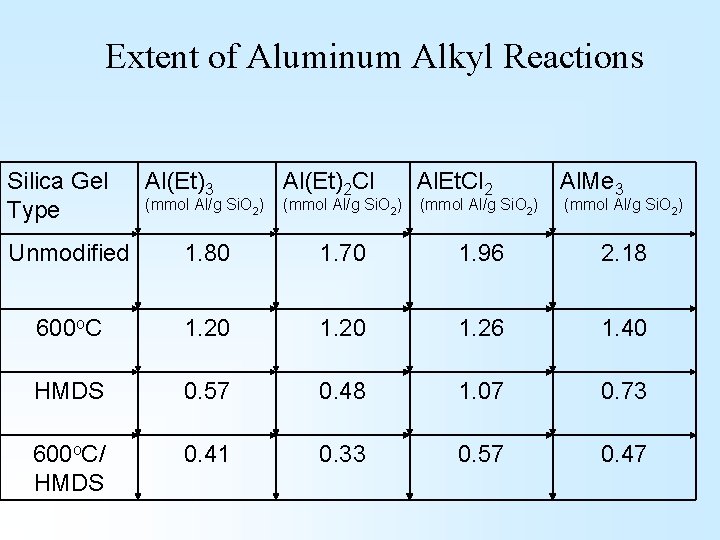
Extent of Aluminum Alkyl Reactions Silica Gel Type Al(Et)3 (mmol Al/g Si. O 2) Al(Et)2 Cl (mmol Al/g Si. O 2) Al. Et. Cl 2 (mmol Al/g Si. O 2) Al. Me 3 (mmol Al/g Si. O 2) Unmodified 1. 80 1. 70 1. 96 2. 18 600 o. C 1. 20 1. 26 1. 40 HMDS 0. 57 0. 48 1. 07 0. 73 600 o. C/ HMDS 0. 41 0. 33 0. 57 0. 47

Extent of Aluminum Alkyl Reactions Silica Gel Type Al(Et)3 (mmol Al/g Si. O 2) Al(Et)2 Cl (mmol Al/g Si. O 2) Al. Et. Cl 2 (mmol Al/g Si. O 2) Al. Me 3 (mmol Al/g Si. O 2) Unmodified 1. 80 1. 70 1. 96 2. 18 600 o. C 1. 20 1. 26 1. 40 HMDS 0. 57 0. 48 1. 07 0. 73 600 o. C/ HMDS 0. 41 0. 33 0. 57 0. 47

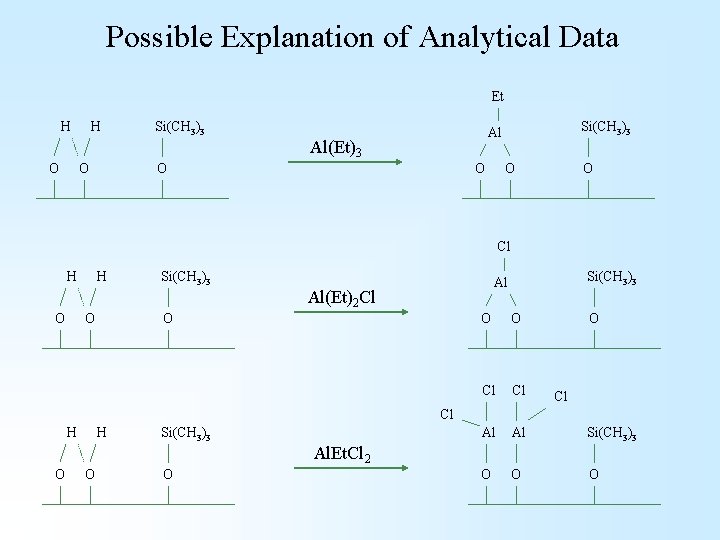
Possible Explanation of Analytical Data Et H H Si(CH 3)3 Al(Et)3 O O Si(CH 3)3 Al O O Cl H H Si(CH 3)3 Al(Et)2 Cl O O Si(CH 3)3 Al O O Cl Cl Al Al Si(CH 3)3 O O O Cl Cl H H Si(CH 3)3 Al. Et. Cl 2 O O O

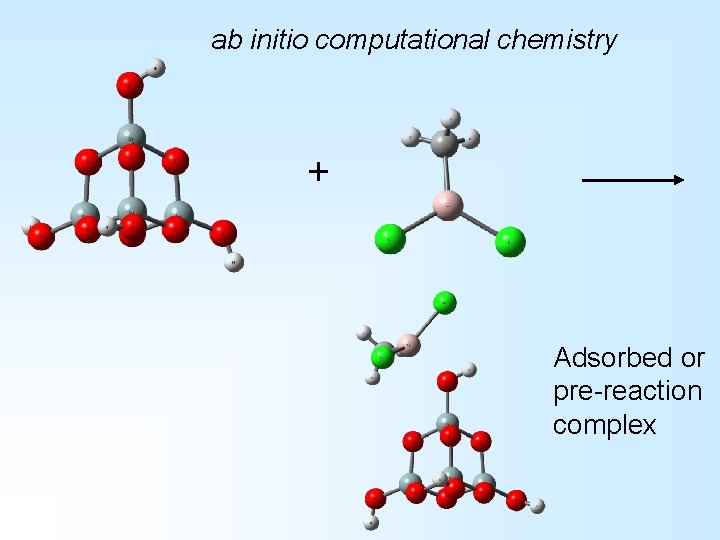
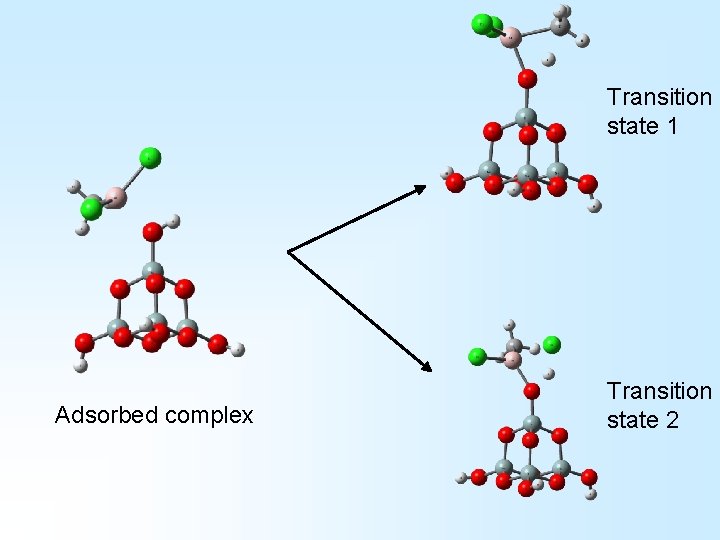
ab initio computational chemistry + Adsorbed or pre-reaction complex

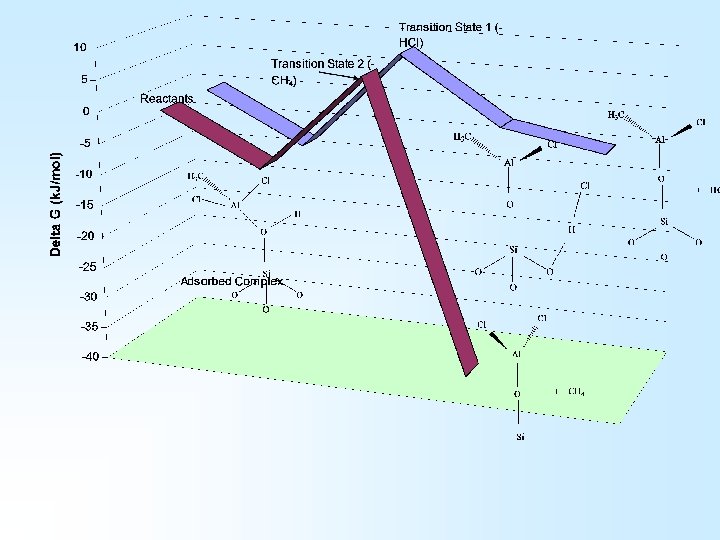
Transition state 1 Adsorbed complex Transition state 2

+ Products 1 + + Products 2 +


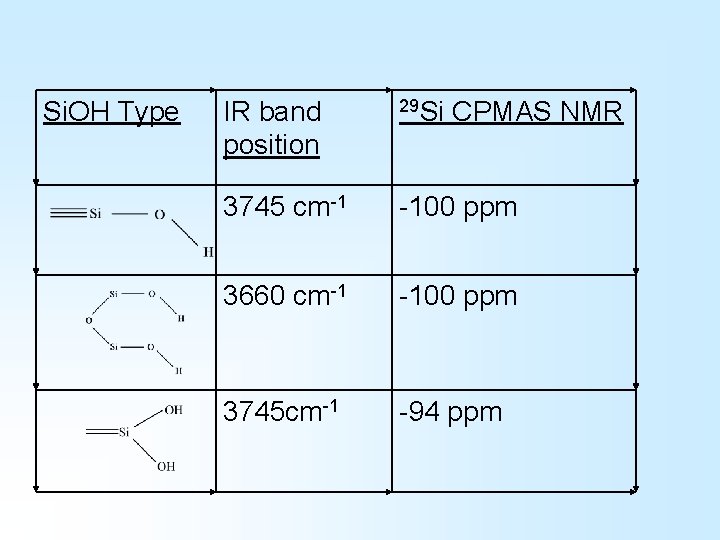
Si. OH Type IR band position 29 Si CPMAS NMR 3745 cm-1 -100 ppm 3660 cm-1 -100 ppm 3745 cm-1 -94 ppm

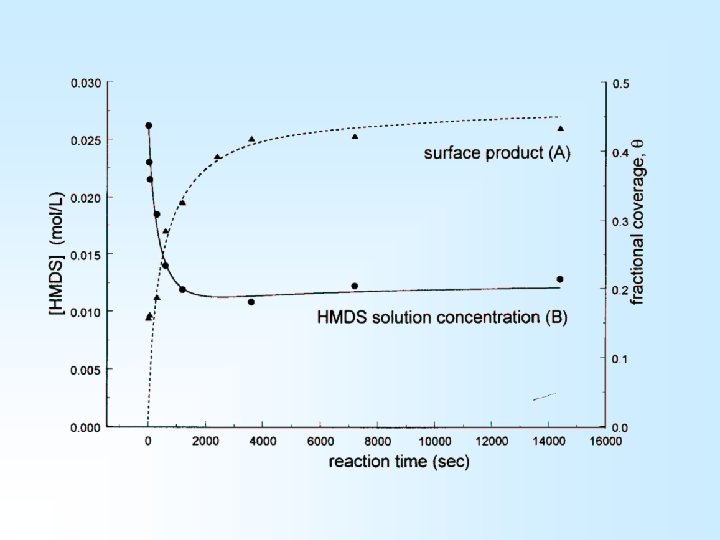
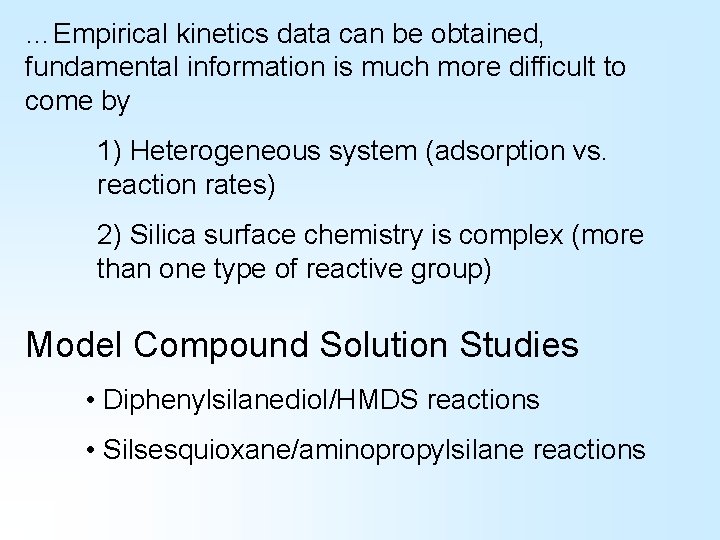
Time is Money • Industrial scale synthesis is aided by reaction kinetics information • Empirical kinetics data can be obtained, fundamental information is much more difficult to come by 1) Heterogeneous system (adsorption vs. reaction rates) 2) Silica surface chemistry is complex (more than one type of reactive group)

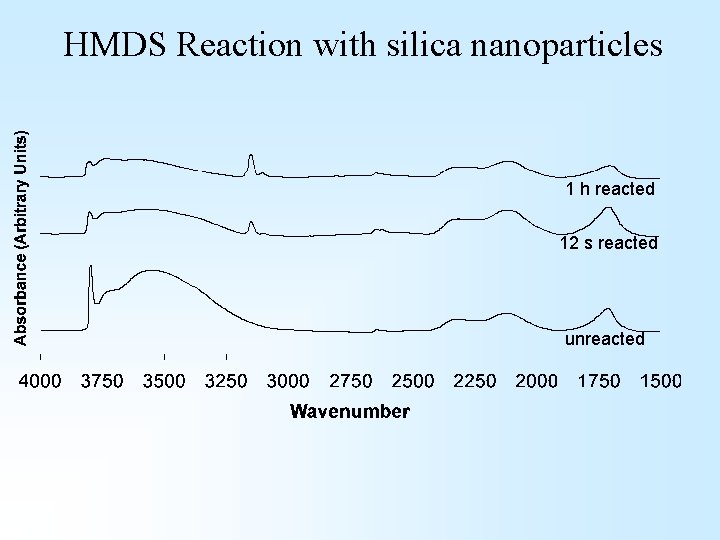
HMDS Reaction with silica nanoparticles 1 h reacted 12 s reacted unreacted

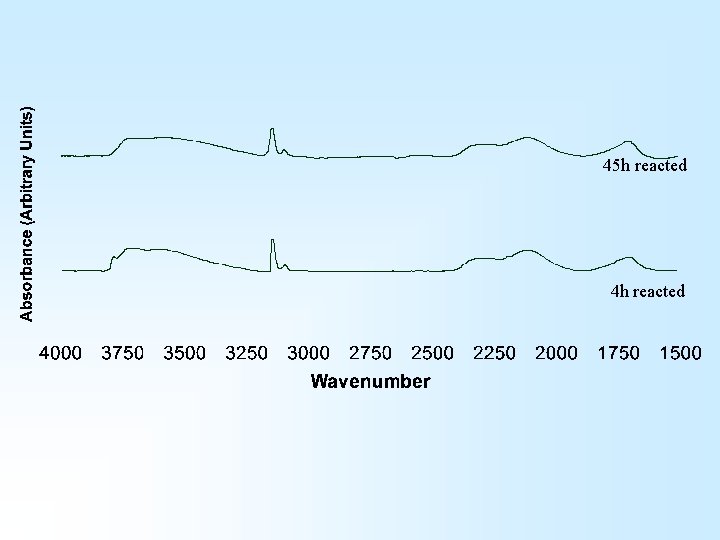
45 h reacted 4 h reacted


Analysis of Kinetics Data • Knowing the reaction mechanism (determined by ab initio calculation)… • Knowing the initial starting conditions, including different silanol concentrations… Differential rate equations for all reactants, transient species, and products obtained. Numerical integration giving best fit to data provides rate constants for various reactions.

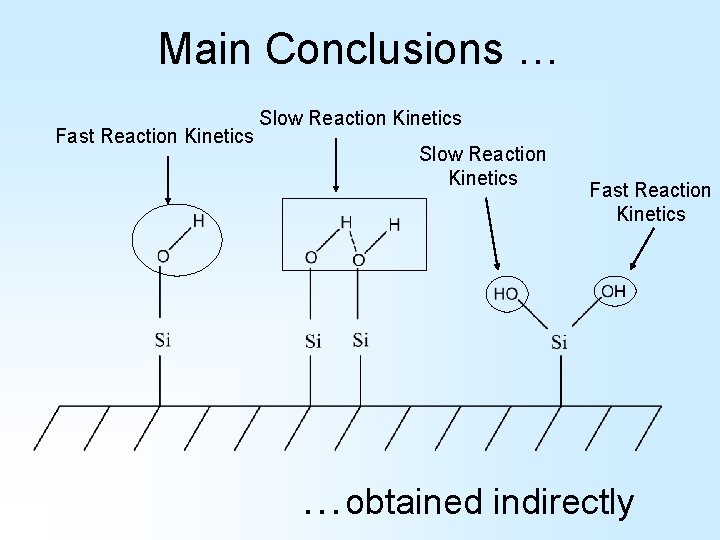
Main Conclusions … Fast Reaction Kinetics Slow Reaction Kinetics Fast Reaction Kinetics …obtained indirectly

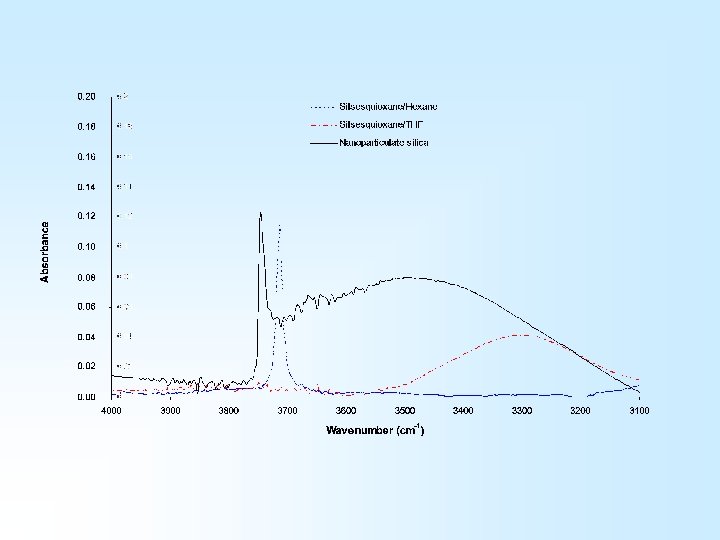
…Empirical kinetics data can be obtained, fundamental information is much more difficult to come by 1) Heterogeneous system (adsorption vs. reaction rates) 2) Silica surface chemistry is complex (more than one type of reactive group) Model Compound Solution Studies • Diphenylsilanediol/HMDS reactions • Silsesquioxane/aminopropylsilane reactions

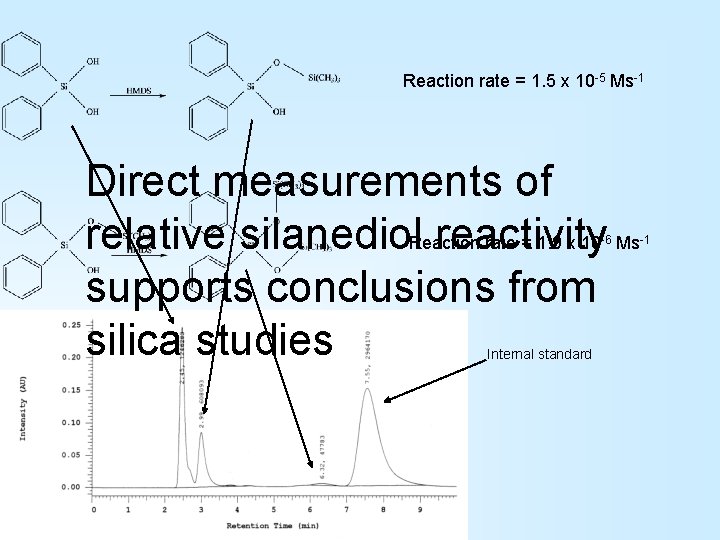
Reaction rate = 1. 5 x 10 -5 Ms-1 Direct measurements of rate = 1. 9 x 10 Ms relative silanediol. Reaction reactivity supports conclusions from silica studies -6 Internal standard -1

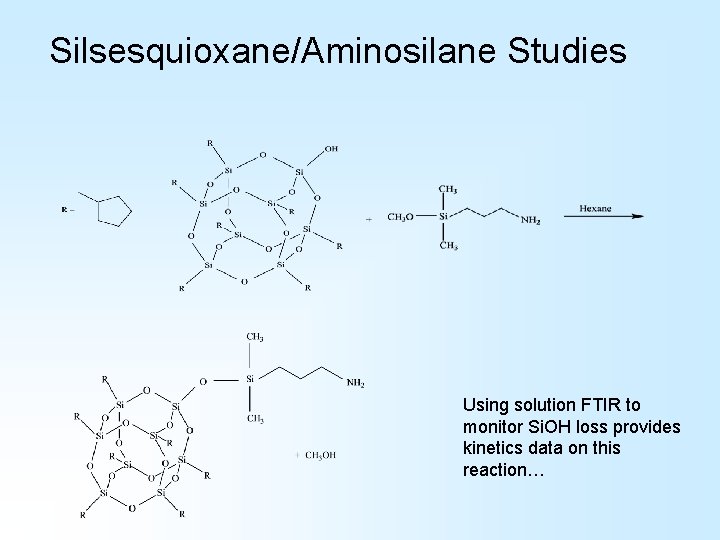
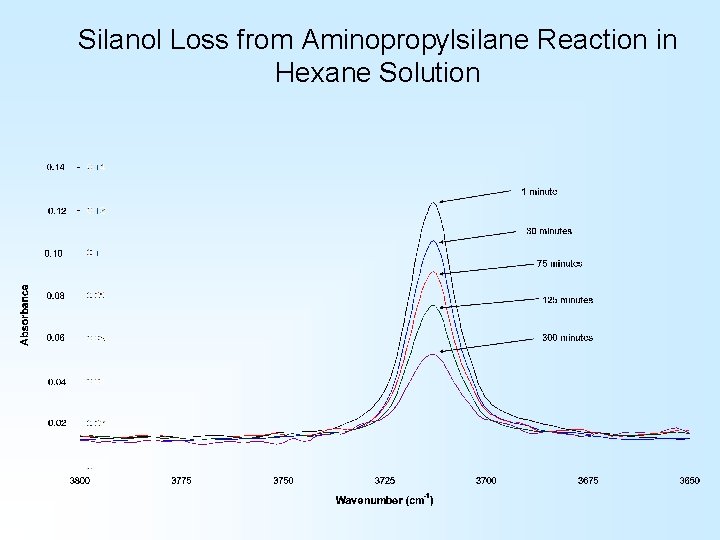
Silsesquioxane/Aminosilane Studies Using solution FTIR to monitor Si. OH loss provides kinetics data on this reaction…

Silanol Loss from Aminopropylsilane Reaction in Hexane Solution



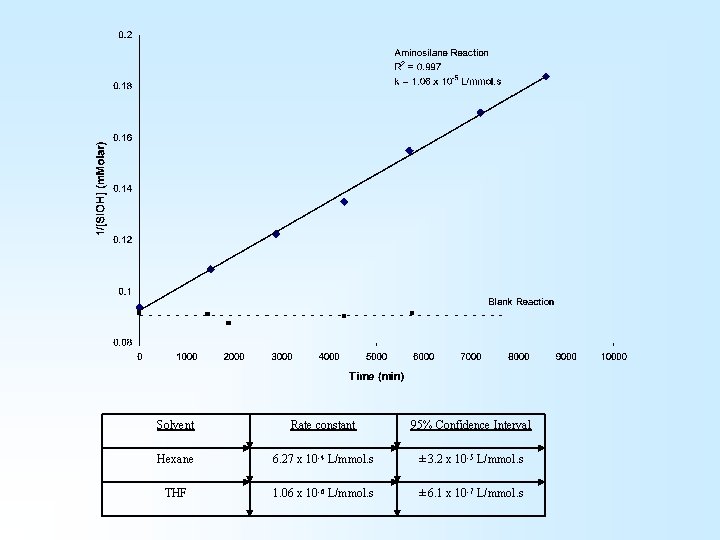
Solvent Rate constant 95% Confidence Interval Hexane 6. 27 x 10 -4 L/mmol. s ± 3. 2 x 10 -5 L/mmol. s THF 1. 06 x 10 -6 L/mmol. s ± 6. 1 x 10 -7 L/mmol. s

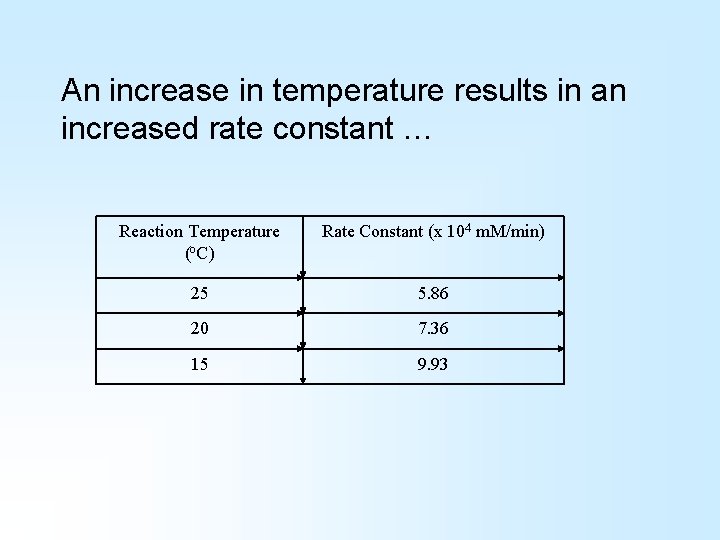
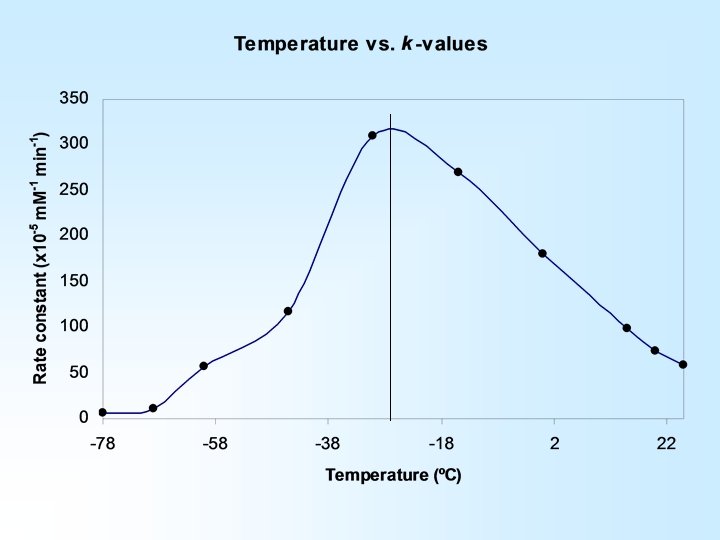
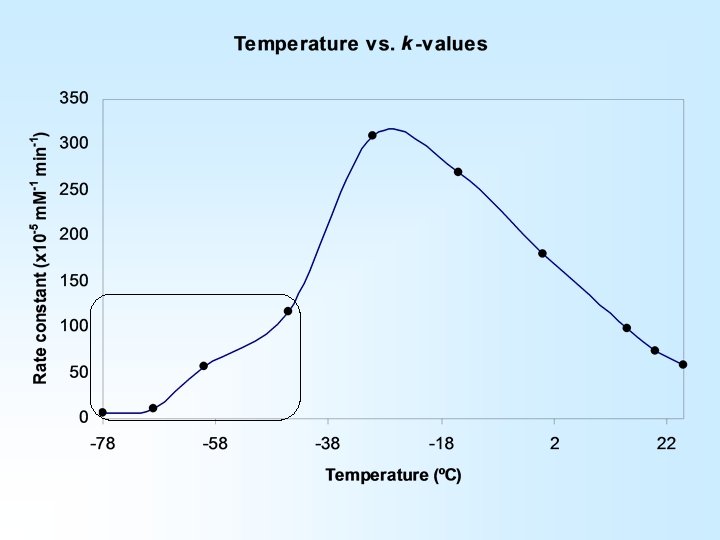
An increase in temperature results in an increased rate constant … Reaction Temperature (o. C) Rate Constant (x 104 m. M/min) 25 5. 86 20 7. 36 15 9. 93


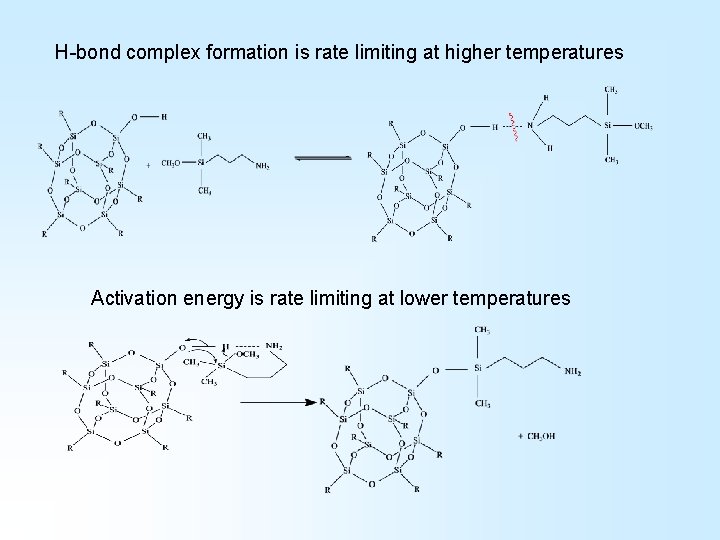
H-bond complex formation is rate limiting at higher temperatures Activation energy is rate limiting at lower temperatures


Arrhenius Plot

Summary • Surface structures can be controlled by silica pretreatments prior to chemical modification • Computational studies provide insight into mechanisms and driving forces • Kinetics data can be obtained on silica – but it is difficult and the information is indirect • Model solution studies provide confirmation and additional information otherwise unobtainable

Acknowledgments Funding: Students & Collaborators: • ACS Petroleum Research Fund • Carol Deakyne • Cabot Corporation • Reto Frei • Equistar Chemicals • Vlad Gun’ko • Eastern Illinois University • Giles Henderson • John Sipple • Mary Vedamuthu