Signs of Chemical Changes 1 2 3 4















- Slides: 25

Signs of Chemical Changes 1. 2. 3. 4. 5. 6.

Chemical change means. . . • Bonds have been ______ • Leads to chemical reactions

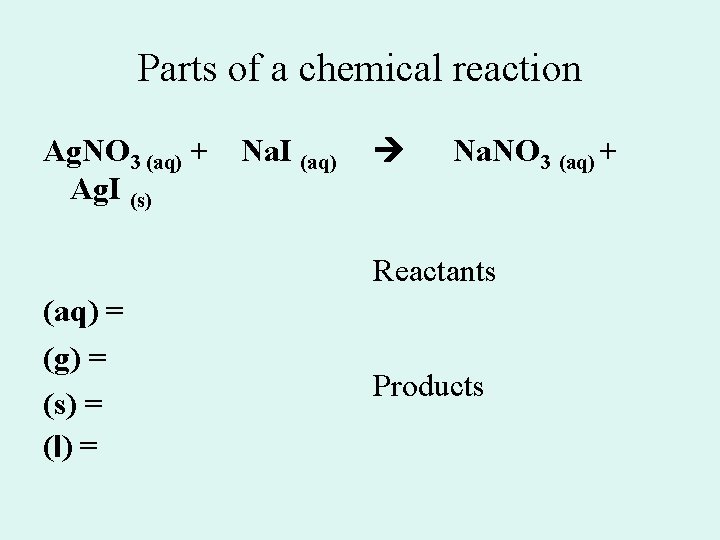
Parts of a chemical reaction Ag. NO 3 (aq) + Ag. I (s) Na. I (aq) Na. NO 3 (aq) + Reactants (aq) = (g) = (s) = (l) = Products

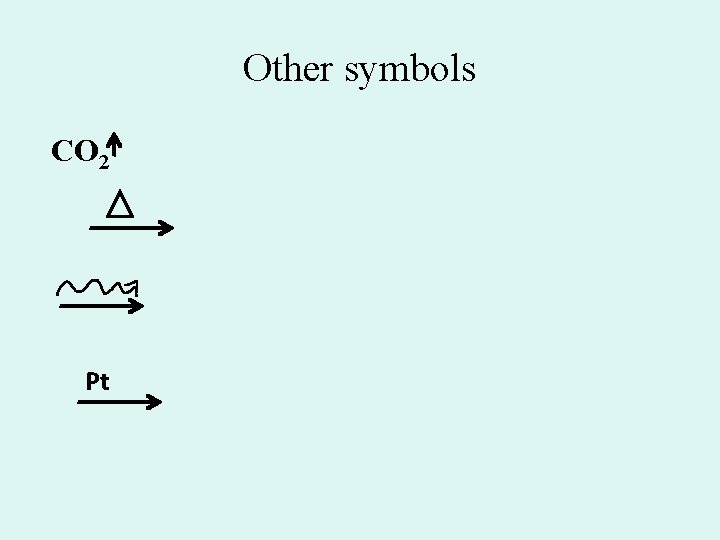
Other symbols CO 2 Pt

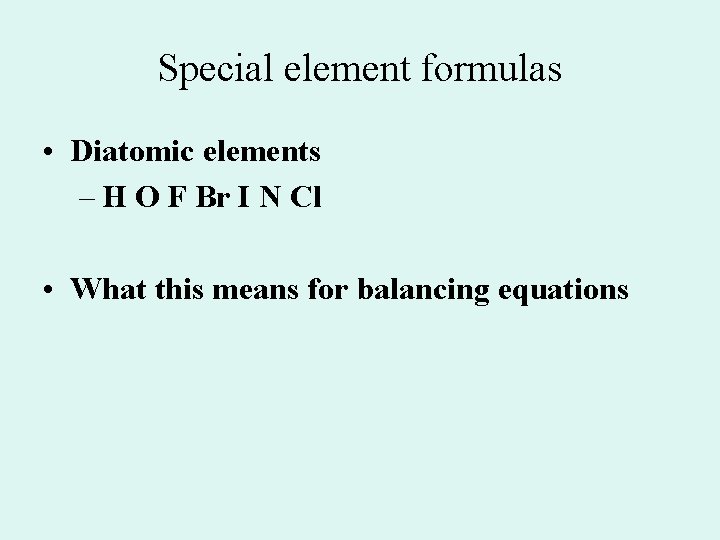
Special element formulas • Diatomic elements – H O F Br I N Cl • What this means for balancing equations

Can’t make something from nothing A loaf of bread has 50 slices (not counting heels) and you have 5 lbs of sliced turkey available. If you are required to put 3. 5 ounces of turkey on a sandwich, what will you run out of first? Show your work to prove your answer.

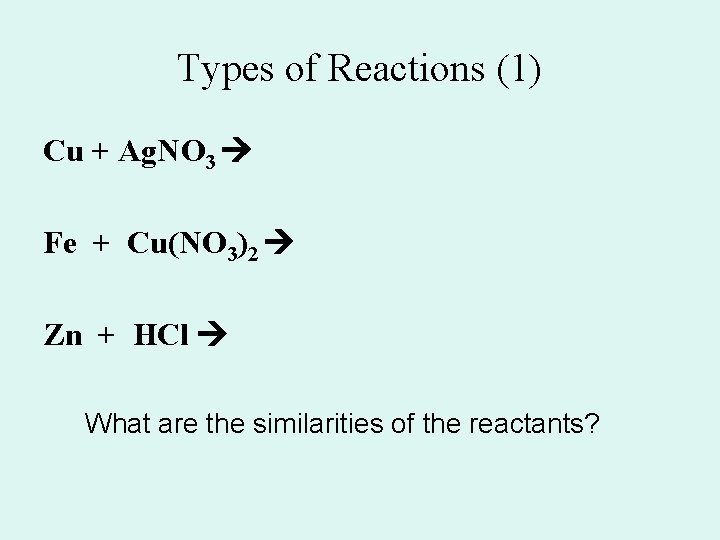
Types of Reactions (1) Cu + Ag. NO 3 Fe + Cu(NO 3)2 Zn + HCl What are the similarities of the reactants?

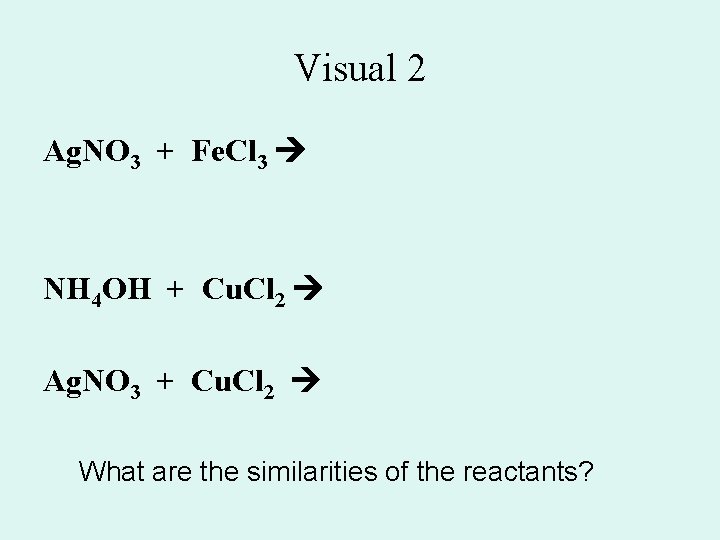
Visual 2 Ag. NO 3 + Fe. Cl 3 NH 4 OH + Cu. Cl 2 Ag. NO 3 + Cu. Cl 2 What are the similarities of the reactants?

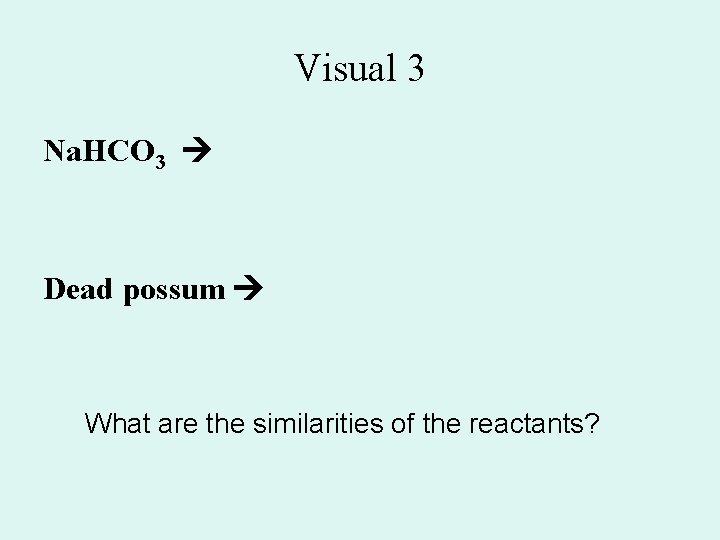
Visual 3 Na. HCO 3 Dead possum What are the similarities of the reactants?

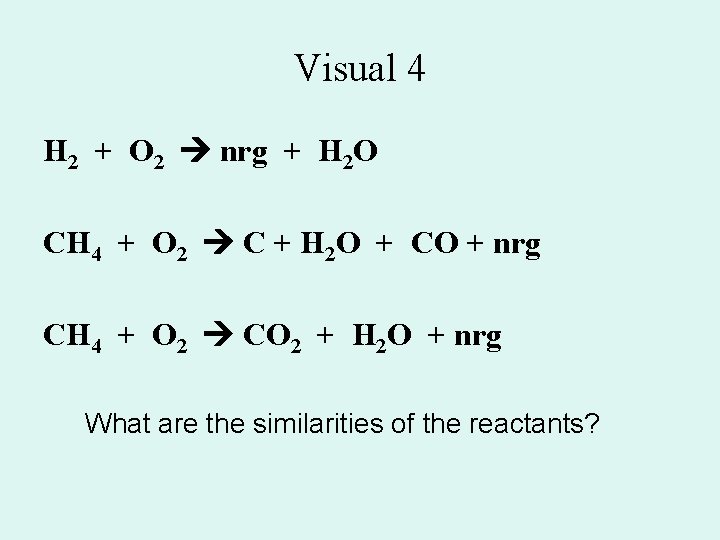
Visual 4 H 2 + O 2 nrg + H 2 O CH 4 + O 2 C + H 2 O + CO + nrg CH 4 + O 2 CO 2 + H 2 O + nrg What are the similarities of the reactants?

A recipe for chocolate cake in my greatgrandmother’s book called for a “short hand of lard. ” How much do you think this is? Why? How is a chili recipe different from a pastry recipe?

Types of Problems Mole to Mass to Mole Mass to Mass

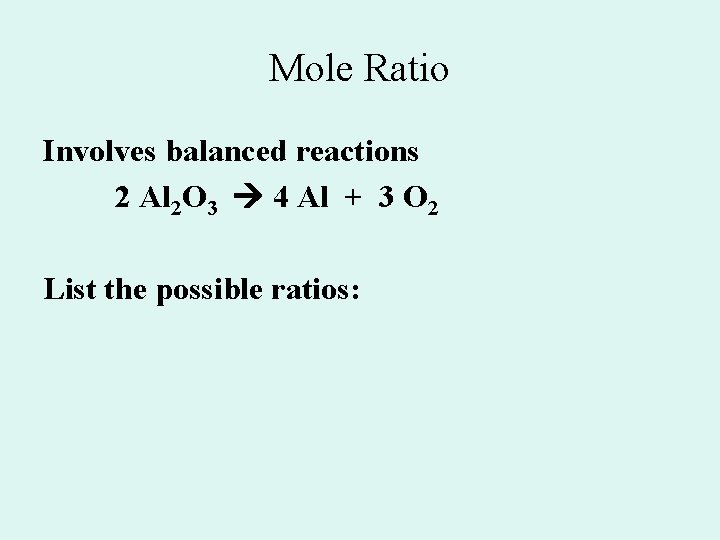
Mole Ratio Involves balanced reactions 2 Al 2 O 3 4 Al + 3 O 2 List the possible ratios:

Practice 1. Mercury (II) oxide decomposes to mercury and oxygen 2. Carbon dioxide + lithium hydroxide yields lithium carbonate + water


Practice wks; you’ll show answers

Mole -- mass Carbon dioxide + water makes C 6 H 12 O 6 and oxygen How many grams of glucose can be made from 3 moles of water?

How to work

Mass to mole NH 3 + oxygen yields nitrogen monoxide and water If 824 g NH 3 are used, how many moles of each product can be formed?

How to work

Mass to Mass Ammonium nitrate is decomposed to make dinitrogen monoxide* and water How many grams of ammonium nitrate are needed to produce 33 g of dinitrogen monoxide? How many grams of water?

How to work


What mass of aluminum is produced by the decomposition of 5. 0 kg of aluminum oxide?

Stoichiometry • Short Google search & pg 803 – What are some of the types of steel? – How are they different? – How is steel made? – How does this NOT fit with stoichiometry?