Significant Figures The first significant figure is the















- Slides: 21

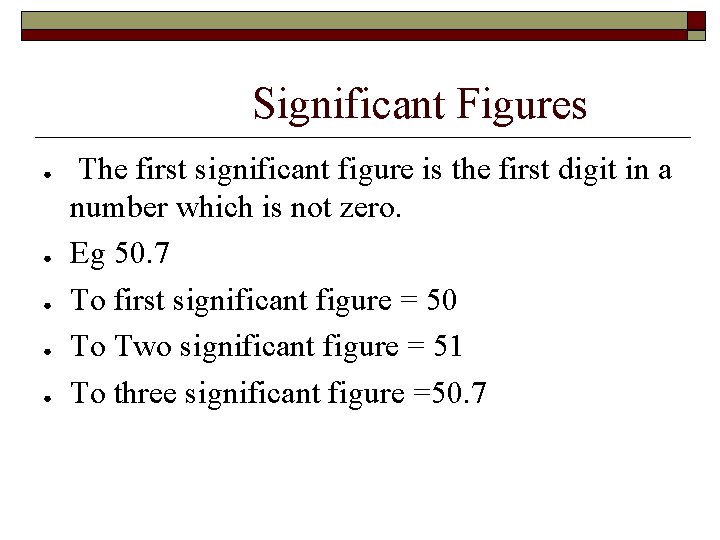
Significant Figures ● ● ● The first significant figure is the first digit in a number which is not zero. Eg 50. 7 To first significant figure = 50 To Two significant figure = 51 To three significant figure =50. 7

Exercise on significant figures. 1. What would you get if you wrote the number 368249 correct to 1 significant figure? 2. What would you get if you wrote the number 0. 00245 correct to 1 significant figure? 3. What would you get if you wrote 0. 0000058763 correct to 2 significant figures? 4. What is 0. 000030456 to two significant figures? 5. What is 7. 994 to two significant figures? 6. How many significant figures does 6559. 060 have?

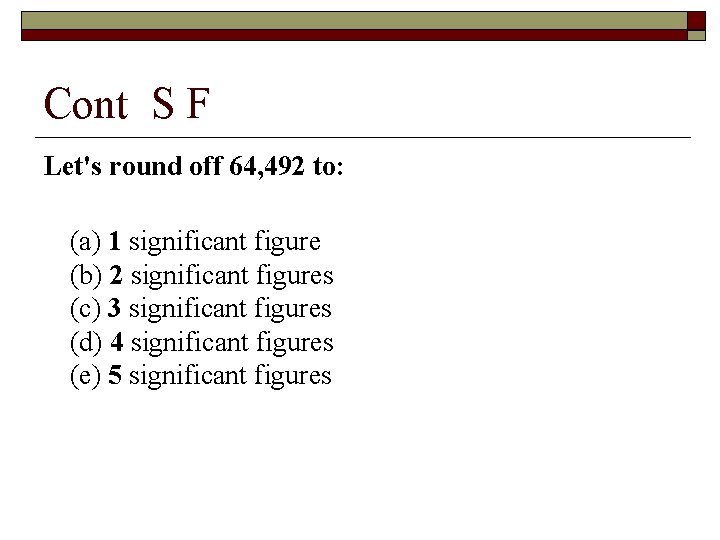
Cont S F Let's round off 64, 492 to: (a) 1 significant figure (b) 2 significant figures (c) 3 significant figures (d) 4 significant figures (e) 5 significant figures

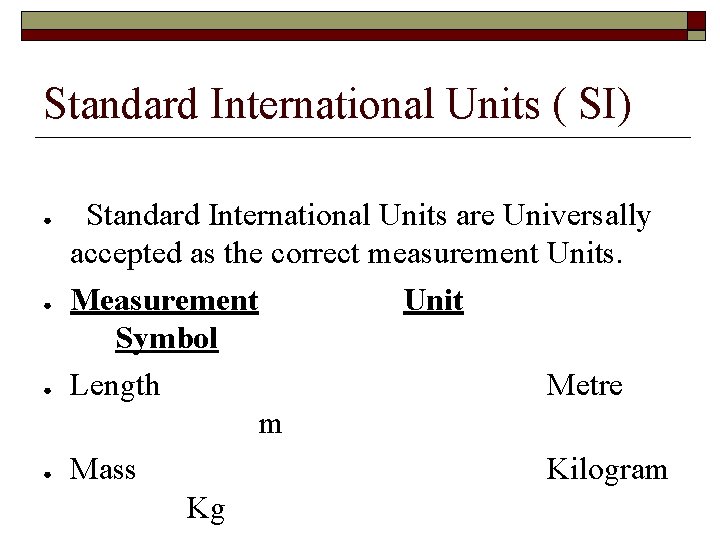
Standard International Units ( SI) ● ● Standard International Units are Universally accepted as the correct measurement Units. Measurement Unit Symbol Length Metre m Mass Kilogram Kg

Continuation In science , we often deal with very large numbers or very small numbers. To deal with these numbers easily, we convert them into standard form.

Continuation In science , we often deal with very large numbers or very small numbers. To deal with these numbers easily, we convert them into standard form.

Standard form cont ● ● A positive superscript number tells you how many places to the right you should move a decimal place. A negative superscript number tells you how many places to the left you should move the decimal point.

GRAMS ● ● ● One gram is equal to one thousand of a Kilogram. 1 Kg= 1000 g 1 g =1 000 ug (microgram) 1. 0 ug =0. 000001 g 2. 0 ug =0. 000002 g 1 mg(milligram) =0. 001 g

Exercise 1) Too much nutmeg can be fatal. The dose is around 20 g. How many mg is this? 2) The average male weighed 8, 400 g what is this in Kg. 3) Most drugs are measured in mg. Paracetamol is 500 mg. How much is this in g. 4) A fish Caught in 1959 weighed 1, 208, 000 mg. What is this in Kg.

Millilitres to Litres 1000 m. L =1 Litre. 1 L = 1000 m. L Exercise 1) 760 m. L. . . . L 2) 8. 1 L. . . . . m. L 3) 41, 000 m. L. . . . L 4) 90, 000 m. L. . . . L

Exercise Cont. 5) 2. 42 L. . . . . m. L 6)5, 210 m. L. . . . . L 7) 0. 4 L. . . . . m. L 8)720 L. . . . . m. L

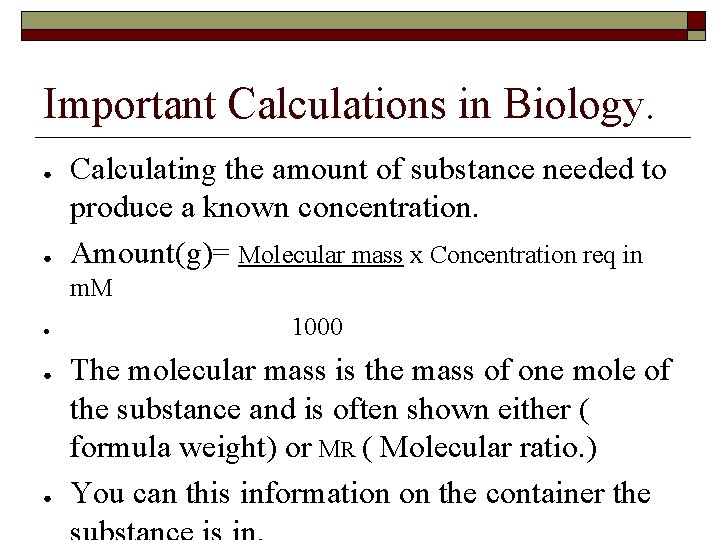
Important Calculations in Biology. ● ● Calculating the amount of substance needed to produce a known concentration. Amount(g)= Molecular mass x Concentration req in m. M ● ● ● 1000 The molecular mass is the mass of one mole of the substance and is often shown either ( formula weight) or MR ( Molecular ratio. ) You can this information on the container the

CONT The result you get from this calculation is PER LITRE of solution so you may need to do some further calculations to decide exactly how much you need. ●

EXAMPLE Calculate the amount of substance needed to produce 1 Litre of 0. 5 Msodium chloride. Na. Cl FW =58. 44 Amount(g) =58. 44 x 500 1000 =29. 22 g

Diluting solution from High Con to Lower concentration. Amount of stock to take= Final Conc x Final Vol Initial(stock) Concentration. ● ● ● This equation is VERY important as it can be used to calculate dilutions REGARDLESS of the starting units. You can dilute substances in moles, % concentration, grams. Etc. Units for final con and stock con MUST be THE SAME.

EXAMPLE You have 1 ml of a 100 mg/ml bromophenol blue solution hat you need to dilute to give 100 ml of a 100 mcg/ml solution.

PIPETTING ● ● ● Pippetting accounts for most of the failed experiments. Pipetting normally affects the accuracy and Precision of an experiment. Precision is the ability to reproduce data. This is mostly expressed as Standard Deviation.

Pipetting Techniques ● ● Setting the Pipette at the correct volume. Tip immersion angle and depth. Aspiration rate. Pre rinse Tip.

SERIAL DILUTION ● ● ● Sometimes we need to measure the concentration of solutions. It may be that the device we use to measure the concentration is only accurate over a certain range. We then dilute our starting stock. Dilution series or serial dilution: Is a set of dilution that reduces the concentration of a substance by a given amount each time.

SERIAL DILUTION ● ● Doubling Dilution : When preparing a doubling dilution, we halve the concentration each time by doubling the Volume.

PRACTICAL See worksheets on serial dilution and serial Dilution.