Significant Figures Significant figures are important in science


- Slides: 8

Significant Figures Significant figures are important in science as they convey uncertainty in measurements and calculations involving measurement. In science, there are no repeating decimal values. Every value has a specific number of digits which are used to express it due to uncertainty in measurement and measuring instruments.

Significant Figures • Significant figures are a simple means for conveying uncertainty associated with a measurement. • They are frequently employed when only one or two measurements are made instead of a series of measurements from which an average (mean) and uncertainty could be determined. • A significant figure or significant digit is one known with reasonable reliability.

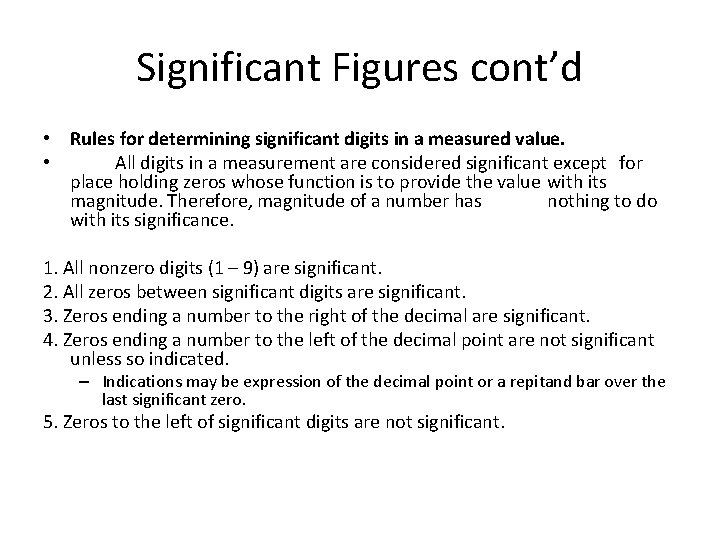
Significant Figures cont’d • Rules for determining significant digits in a measured value. • All digits in a measurement are considered significant except for place holding zeros whose function is to provide the value with its magnitude. Therefore, magnitude of a number has nothing to do with its significance. 1. All nonzero digits (1 – 9) are significant. 2. All zeros between significant digits are significant. 3. Zeros ending a number to the right of the decimal are significant. 4. Zeros ending a number to the left of the decimal point are not significant unless so indicated. – Indications may be expression of the decimal point or a repitand bar over the last significant zero. 5. Zeros to the left of significant digits are not significant.

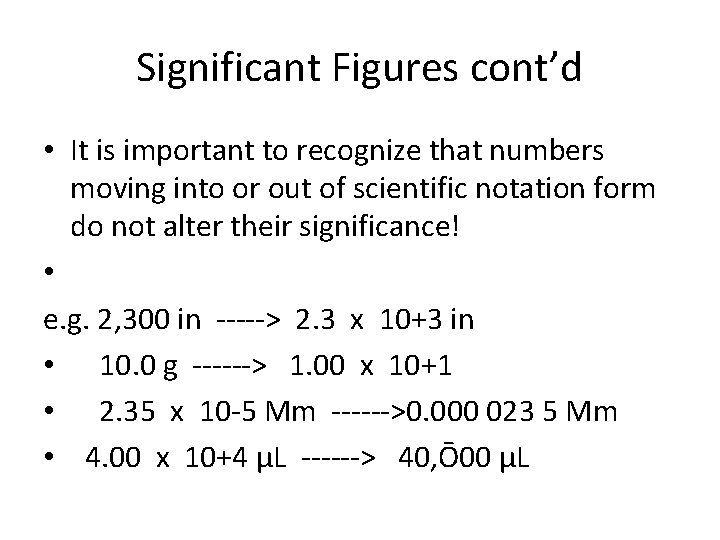
Significant Figures cont’d • It is important to recognize that numbers moving into or out of scientific notation form do not alter their significance! • e. g. 2, 300 in -----> 2. 3 x 10+3 in • 10. 0 g ------> 1. 00 x 10+1 • 2. 35 x 10 -5 Mm ------>0. 000 023 5 Mm • 4. 00 x 10+4 μL ------> 40, Ō00 μL

MAKE A NOTE!! • NOTE: All problems given on tests and quizzes in this class will be expressed to the desired significance. Treat them accordingly!!!

Operations Using Significant Figures • Addition and subtraction • When adding or subtracting, identify the least significant value on a units/decimal place basis. – Value with largest uncertainty • Perform the operation (addition or subtraction). • Round the result to the same units/decimal place as the least significant value.

Operations Using Significant Figures • Multiplication and division • When multiplying or dividing, identify the least significant value based upon the number of significant digits. • Perform the operation (multiplying or dividing). • Round the result to have the same number of significant digits as the least significant figure.

Rules for Rounding • 1. If the first digit dropped is less than 5, leave the preceding number unchanged. (3. 123 becomes 3. 12) • 2. If the first digit dropped is greater than 5, increase the preceding digit by 1. (3. 127 becomes 3. 13) • 3. If the first digit dropped is exactly 5, round off to make the preceding digit an even number. (4. 125 becomes 4. 12, but 4. 135 becomes 4. 14) – Exactly 5, (or 1/2), means there could be any number of zeros after the five, however, no nonzero digits.