Significant Figures sig figs Measuring volume SigFig Rules























































- Slides: 59

Significant Figures (sig figs)

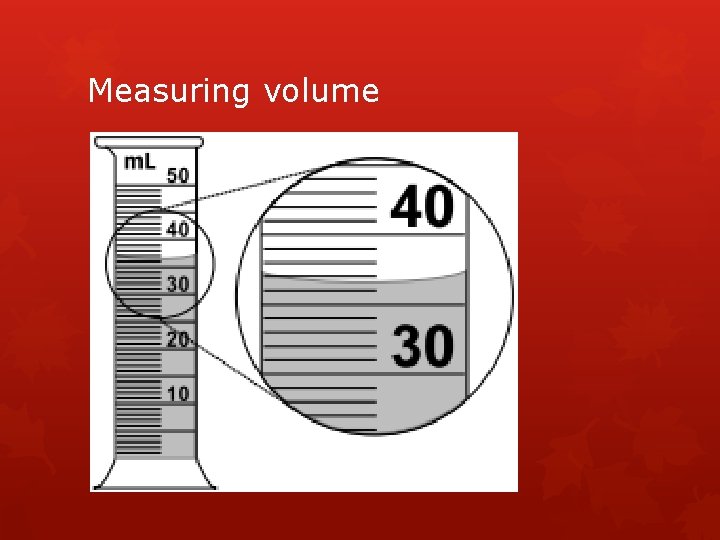
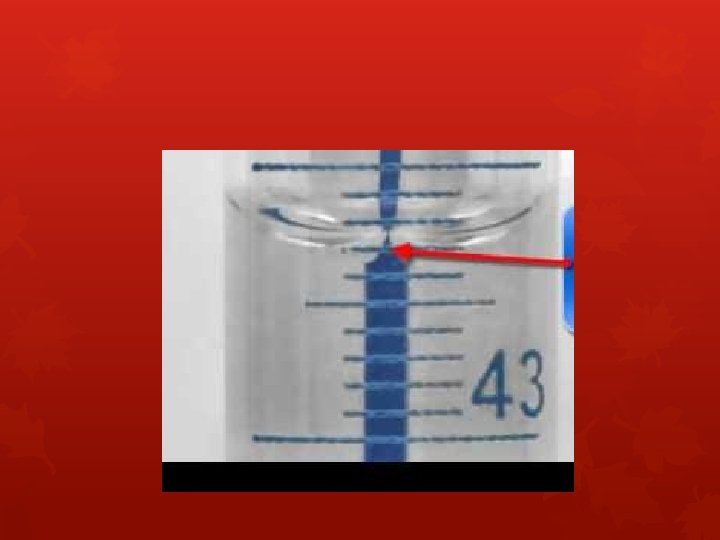
Measuring volume



Sig-Fig Rules For measured values…all certain digits +1 estimated digit. 1. All non-zero digits are significant Example: 2. 4 (2 sig figs) 2. Leading zeros are NEVER significant Example: 0. 01 (1 sig fig) 3. In between zeros are always significant Example: 2. 017 (4 sig figs) 4. Trailing zeros are significant if there is a decimal point in the number Example: 0. 00200200 (6 sig figs)

Extra Rules/tips In scientific notation, only count the digits in the coefficient

Exact Numbers Exact numbers, such as the number of people in a room, have an infinite number of significant figures. Another example of this are defined numbers, such as 1 foot = 12 inches. There are exactly 12 inches in one foot. Therefore, if a number is exact, it DOES NOT affect the accuracy of a calculation nor the precision of the expression.

Practice 0. 00100 m 2. 08 x 102 L 480 cars 6, 280 feet 9000 inches 9000. inches 1 quart = 0. 946 L

Addition and subtraction Answer is rounded to the least amount of decimal places Example: 5. 02 - 3. 486 +5. 1 =

Rounding If the digit is less than 5, round down. If the digit is equal or greater than 5, round up

Multiplication and Division Final answer sig figs depends on the value with lowest number of sig figs in the problem. Example: 6. 56 x 2. 3 Number of sig figs? Answer =______

Conclusion Why can you not simply put the answer your calculator gives you when adding and subtracting? When adding and subtracting, what are you basing your answer on so your answer reflects the precision of your measurements? When multiplying and dividing, what are you basing your answer on so your answer reflects the precision of your measurements?

Review I have 425 grams of fruit loops. If 15 fruit loops is 3. 0 grams, how many fruit loops do I have?

Extended Practice Problem An empty graduated cylinder has a mass of 15. 31 g. When 8. 357 m. L of an unknown liquid is placed in the graduated cylinder, the combined mass is found to be 24. 86 g. What is the density of the liquid?

PERIODIC TABLES!!!!

HOMEWORK! Sig figs practice problems.

The Mole…. . What do the substances on the front table have in common?

BREAKING BAD $43, 860, 000.

Essential Question: How many atoms are in “that”? Notice “that in quotation marks” What does this mean?

THE MOLE…. . Atoms are very small. In order to quantify them, we need a lot, 6. 02 x 1023 to be exact 6. 02 x 1023 of anything per mole Example. 1 mole of paperclips= 6. 02 x 1023 paperclips How many baseballs are in 1 mole of baseballs?

THE MOLE…. . The periodic table gives us mass in grams per mole, which means the mass of 6. 02 x 1023 atoms each element Moles are used as a conversion factor (think…. g/mol)

Finding molecular weights of compounds Write out each element in the compound. Supply the molecular weight for each compound, as given on the periodic table Multiply each molecular weight by the subscript as given in the compound. Add them up. Ex. H 3 PO 4 H= P= O= =

Carbon

Potassium

Lithium

Beryllium

Magnesium

Manganese

Manganese

Fluorine

P

Na

P

Si

Sn

Bi

Au

Sb

U

Copper

Zinc

Iron

Titanium

Neon

Ca

S

I

Ar

Mercury

Silver

Review If I have one mole of beef jerky sticks, how many beef jerky sticks do I have?

MOLE HIGHWAY! Volume MASS MOLECULES https: //www. youtube. com/watch? v=Kg-za. G 0 ck. Vg

DO NOW! What is the molar mass of Ag. Cl? If I have 4. 0 grams of Ag. Cl, how many moles do I have? How many molecules of Ag. Cl do I have? If I have 4. 0 grams of Ag. Cl, how many molecules do I have?

Essential Question! How “strong” is that solution? Looking at the three solutions on the front table, what is the difference between the three?

Essential Question! How “strong” is that solution? Looking at the three solutions on the front table, what is the difference between the three? Think about concentrated vs. dilute

Concentrated vs. Dilute With a partner, come up with a definition of the following terms. Concentrated: Dilute:

What you will be doing…. . Differentiate between different methods of describing the concentration of a solution. Given two of three characteristics of volume, molarity, and solute mass, calculate third. Prepare a solution of given concentration. Calculate the effects of colligative properties of a solution.

Molarity How we define the concentration of a solution Use the equation: Molarity (M) = mol solute/liters of solution Review: What is the solute? What is the solvent? Let’s Practice. Get out your workbooks and turn to Guided Practice: Molarity of Solutions

MOLE HIGHWAY! Volume MASS MOLECULES