Significant Figures Introduction Using the scale below measure
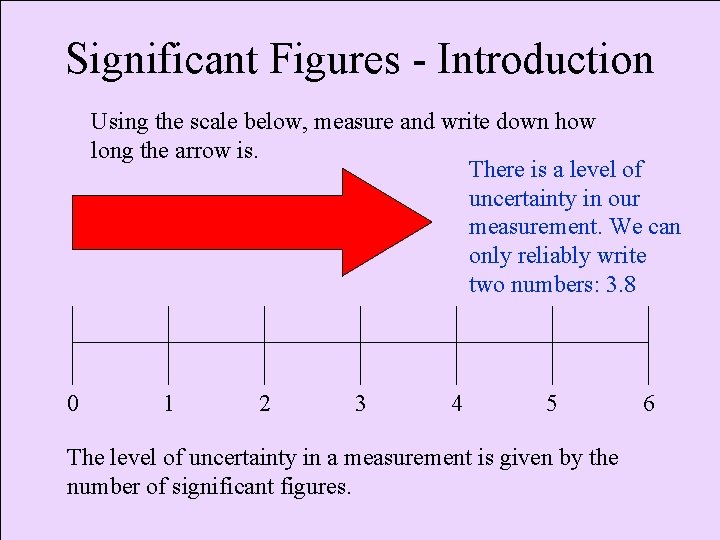
Significant Figures - Introduction Using the scale below, measure and write down how long the arrow is. There is a level of uncertainty in our measurement. We can only reliably write two numbers: 3. 8 0 1 2 3 4 5 The level of uncertainty in a measurement is given by the number of significant figures. 6

Rules for Significant Figures • A trailing zero is significant: 4. 130 • 4 significant figures • A zero within a number is significant: 35. 06 • 4 significant figures • A zero before a digit is not significant: 0. 082 • 2 significant figures • A number ending in zero with no decimal point, as in 20, is ambiguous. • 1 or 2 significant figures

Calculations using Significant Figures • Multiplication and division: – The number of significant figures is determined after all the calculations and is equal to the smallest number of sig figs in the original numbers. • Example 4. 135 x 2. 4 = 9. 924 4. 135 cm x 2. 4 cm = But we can only use 2 sig figs Round 9. 924 to 9. 9 = 9. 9 cm 2

Calculations using Sig. Figs. • Addition and Subtraction – After finishing the calculation, the number of sig figs after the decimal point must be equal to the number of sig figs in the least well known original number • Example 4. 5 mm - 1. 258 mm = -1. 258 2 3. 242 But the decimal that is uncertain (least well known) is the first decimal place So round 3. 242 to 3. 2 4. 5 mm - 1. 258 mm = 3. 2 mm

Physical or Chemical • Physical Change - the compound undergoes a change without losing its identity – Freezing, melting, evaporation, condensation • Chemical Change - the compound reacts with another substance and loses its identity to become a new substance – Burning gasoline, Baking a cake
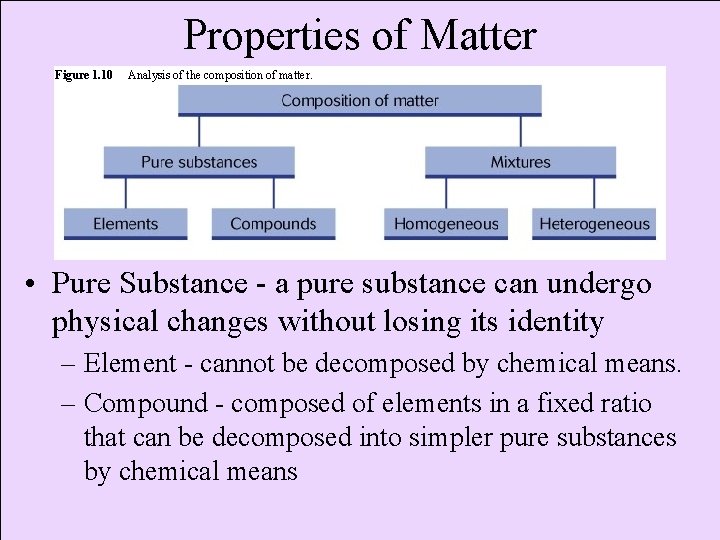
Properties of Matter Figure 1. 10 Analysis of the composition of matter. • Pure Substance - a pure substance can undergo physical changes without losing its identity – Element - cannot be decomposed by chemical means. – Compound - composed of elements in a fixed ratio that can be decomposed into simpler pure substances by chemical means
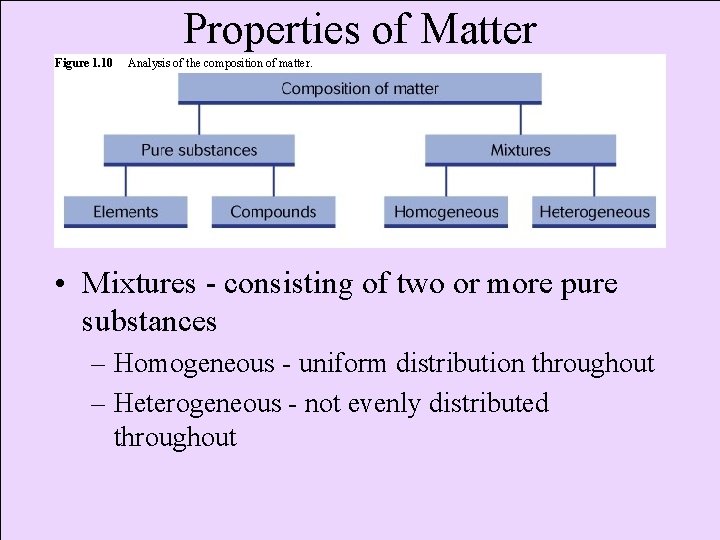
Properties of Matter Figure 1. 10 Analysis of the composition of matter. • Mixtures - consisting of two or more pure substances – Homogeneous - uniform distribution throughout – Heterogeneous - not evenly distributed throughout
Fundamental Properties of Matter • Mass - the measure of the quantity of matter (in kg) – different than weight - the response of mass to gravity • Think of yourself on the moon vs. on Earth • Volume - amount of space that a sample occupies (in L)

Q: Metal sinks in water, but a boat, made out of metal, floats. How can this be? • Answer: Density. – Density (d) = mass (g)/ volume (cm 3 or m. L) • When something is less dense than water, it floats. Aluminum boat mass = 5. 35 x 103 g Volume of empty Boat = 1. 8 x 104 m. L Density = 5. 35 x 103 g/1. 8 x 104 m. L = 0. 29 g/m. L Because a boat is mostly hollow, its density is less than water so it floats.
- Slides: 9