Significant Figures and Scientific Notation What is a











- Slides: 21

Significant Figures and Scientific Notation

What is a Significant Figure? There are 2 kinds of numbers: ØExact: the amount of money in your account. Known with certainty. ØApproximate: weight, height— anything MEASURED. No measurement is perfect.

What is a Significant Figure? ØThe numbers reported in a measurement are limited by the measuring tool ØSignificant figures in a measurement include the known digits plus one estimated digit

Using Significant Figures ØWhen a measurement is recorded only those digits that are dependable are written down. ØThe numbers reported in a measurement are limited by the measuring tool

Rules for Significant Figures 1. All non-zero digits in a number are significant 2. Zeros between nonzero numbers are significant 41, 026 32. 001 Count these

Learning Check How many Significant Figures? 7. 16 25 1. 9648 43. 104 2. 0003

Rules for Significant Figures 3. Trailing zeros are not significant 8100 3, 600, 000 4. All numbers after a decimal point are significant except for leading zeros 0. 365 0. 00972 Do not count these

Learning Check How many Significant Figures? 34, 500 28. 077 1, 600 0. 039 34, 500. 0

Sig Figs in Scientific Notation 5. All numbers in scientific notation are significant. When writing scientific notation, do not write a number that is not meant to be a sig fig. 3. 2 x 103 2. 00 x 10 -4

Learning Check How many Significant Figures? 2. 62 x 10 -2 1. 0 x 102 9. 7000 x 108 5 x 104 3 x 10 -6

Sig Figs in Calculations ØA calculated answer cannot be more precise than the measuring tool. ØA calculated answer must match the least precise measurement. ØSignificant figures apply to final answers from 1) adding or subtracting 2) multiplying or dividing 11

Adding and Subtracting The answer has the same number of decimal places as the measurement with the fewest decimal places. 25. 2 one decimal place + 1. 34 two decimal places 26. 54 answer 26. 5 one decimal place

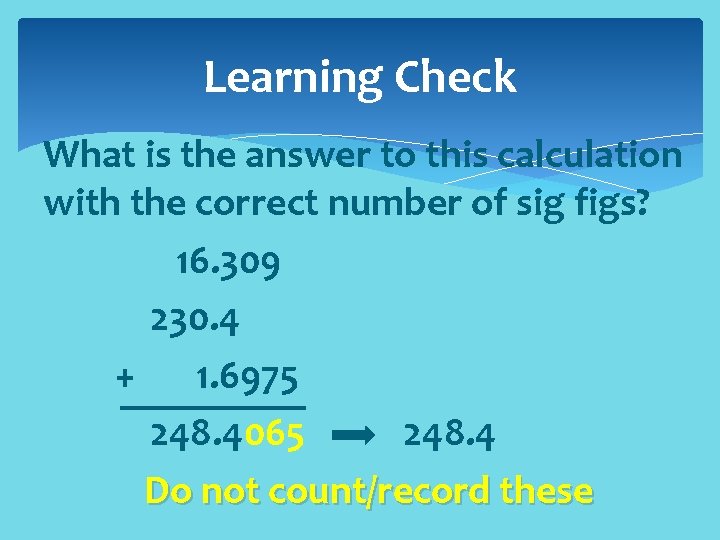
Learning Check What is the answer to this calculation with the correct number of sig figs? 16. 309 230. 4 + 1. 6975 248. 4065 248. 4 Do not count/record these

Multiplying and Dividing The answer has the same number of sig figs as the least precise measurement. Round the answer to this number. 4. 15 3 sig figs x 20 1 sig fig 83 must round to 1 sig fig 80

Learning Check What is the answer to this calculation with the correct number of sig figs? 2. 54 x 0. 0028 = 11. 2888889 0. 0105 x 0. 060 Round to 2 sig figs 11 Do not count these

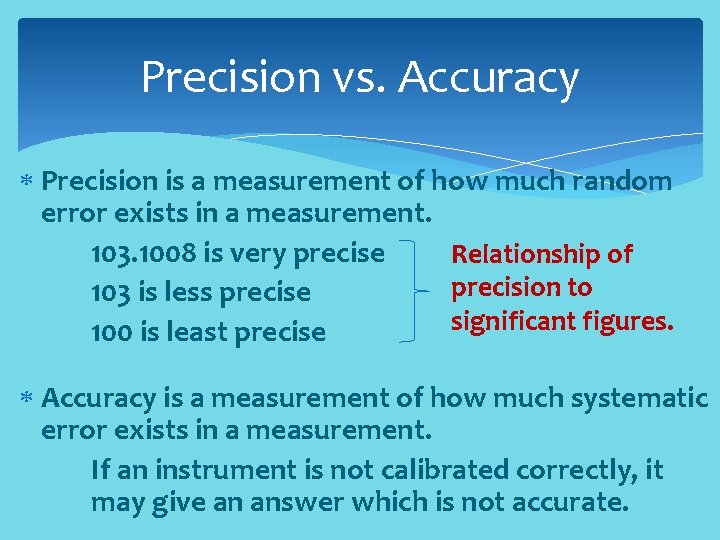
Precision vs. Accuracy Precision is a measurement of how much random error exists in a measurement. 103. 1008 is very precise Relationship of precision to 103 is less precise significant figures. 100 is least precise Accuracy is a measurement of how much systematic error exists in a measurement. If an instrument is not calibrated correctly, it may give an answer which is not accurate.

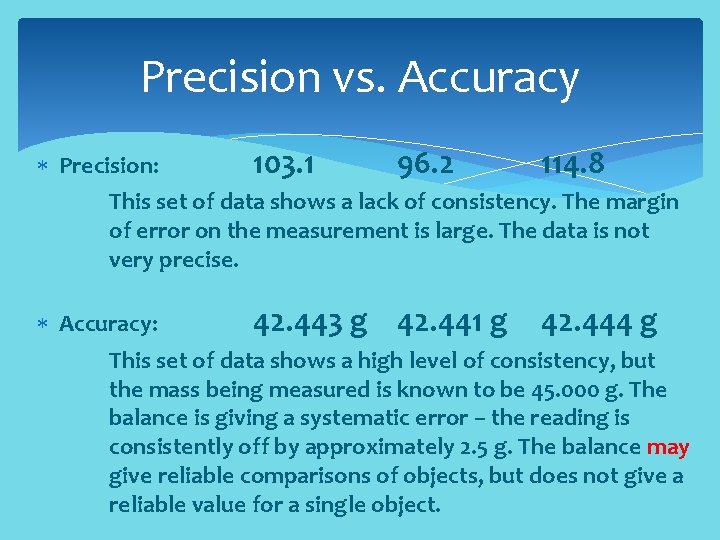
Precision vs. Accuracy Precision: 103. 1 96. 2 114. 8 This set of data shows a lack of consistency. The margin of error on the measurement is large. The data is not very precise. Accuracy: 42. 443 g 42. 441 g 42. 444 g This set of data shows a high level of consistency, but the mass being measured is known to be 45. 000 g. The balance is giving a systematic error – the reading is consistently off by approximately 2. 5 g. The balance may give reliable comparisons of objects, but does not give a reliable value for a single object.

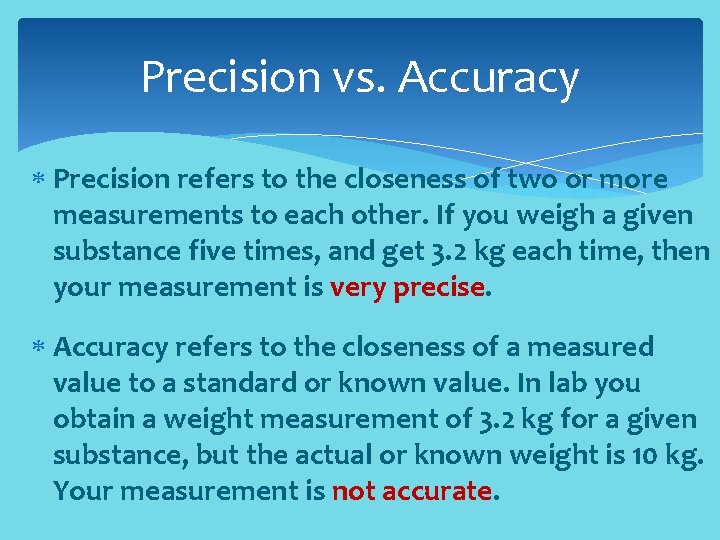
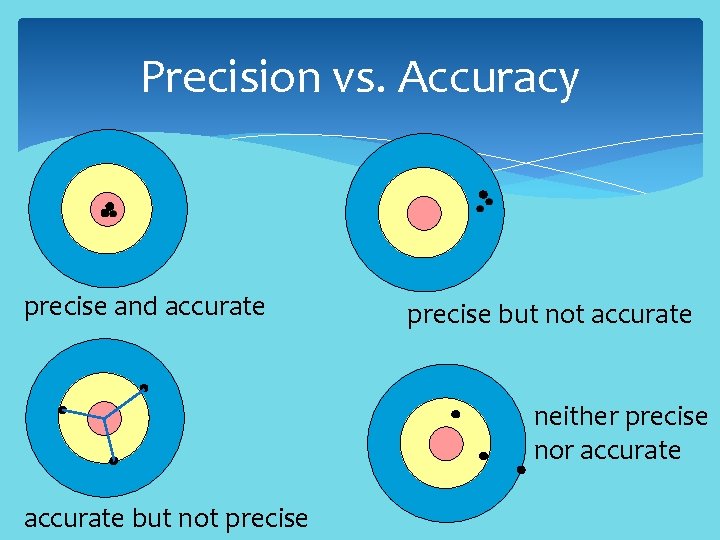
Precision vs. Accuracy Precision refers to the closeness of two or more measurements to each other. If you weigh a given substance five times, and get 3. 2 kg each time, then your measurement is very precise. Accuracy refers to the closeness of a measured value to a standard or known value. In lab you obtain a weight measurement of 3. 2 kg for a given substance, but the actual or known weight is 10 kg. Your measurement is not accurate.

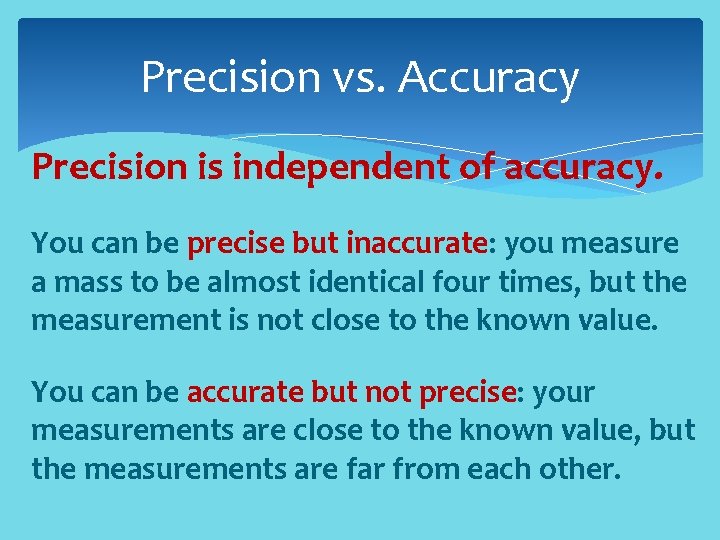
Precision vs. Accuracy Precision is independent of accuracy. You can be precise but inaccurate: you measure a mass to be almost identical four times, but the measurement is not close to the known value. You can be accurate but not precise: your measurements are close to the known value, but the measurements are far from each other.

Precision vs. Accuracy precise and accurate precise but not accurate neither precise nor accurate but not precise

Precision vs. Accuracy A good analogy for understanding accuracy and precision is to imagine a basketball player shooting baskets. If the player shoots with accuracy, his aim will take the ball close to or into the basket. If the player shoots with precision, his aim will always take the ball to the same location which may or may not be close to the basket. A good player will be both accurate and precise by shooting the ball the same way each time and each time making it in the basket.