Significant Digits or Figures How to recognize significant


















- Slides: 31

Significant Digits or “Figures” • How to recognize significant figures when: – Taking a measurement – Reading a measurement – Performing a calculation

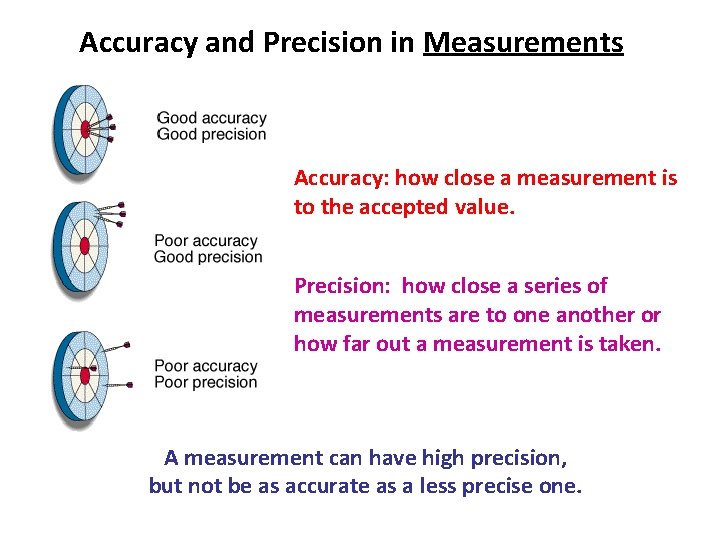
Accuracy and Precision in Measurements Accuracy: how close a measurement is to the accepted value. Precision: how close a series of measurements are to one another or how far out a measurement is taken. A measurement can have high precision, but not be as accurate as a less precise one.

Significant Figures are used to indicate the precision of a measured number or to express the precision of a calculation with measured numbers. In any measurement the digit farthest to the right is considered to be estimated. 0 1 2 1. 3 2. 0

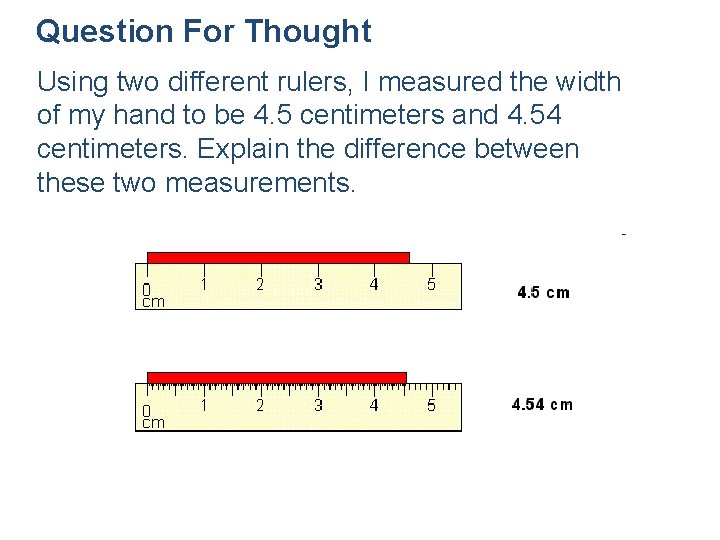
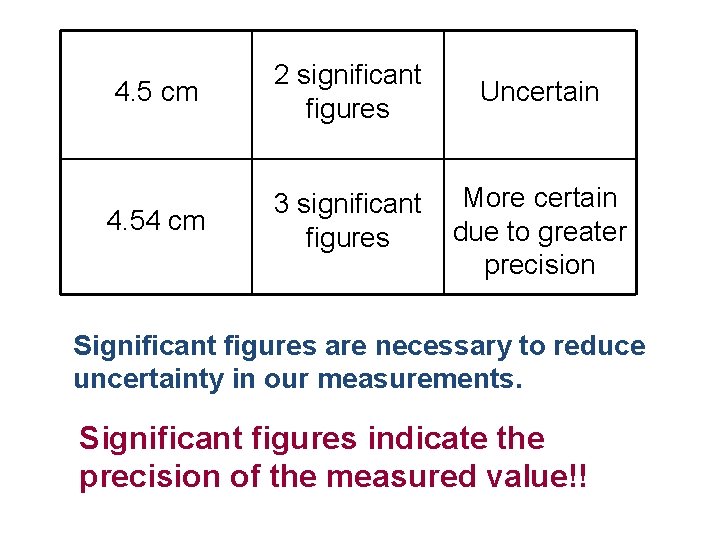
Question For Thought Using two different rulers, I measured the width of my hand to be 4. 5 centimeters and 4. 54 centimeters. Explain the difference between these two measurements.

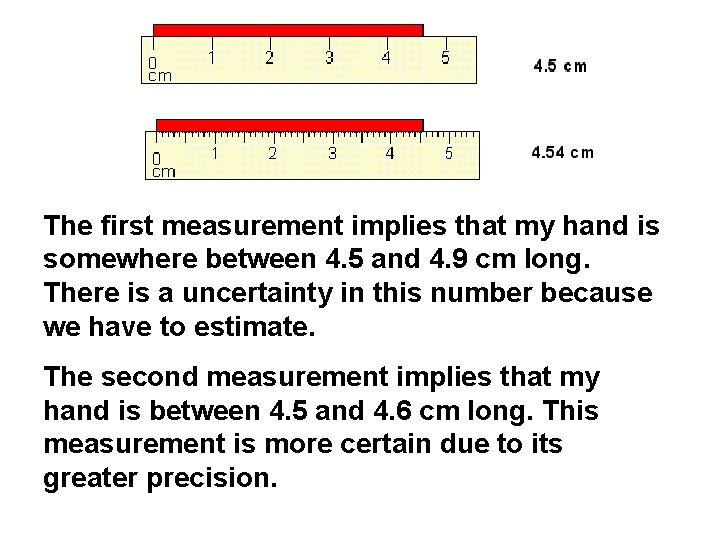
The first measurement implies that my hand is somewhere between 4. 5 and 4. 9 cm long. There is a uncertainty in this number because we have to estimate. The second measurement implies that my hand is between 4. 5 and 4. 6 cm long. This measurement is more certain due to its greater precision.

4. 5 cm 2 significant figures Uncertain 4. 54 cm 3 significant figures More certain due to greater precision Significant figures are necessary to reduce uncertainty in our measurements. Significant figures indicate the precision of the measured value!!

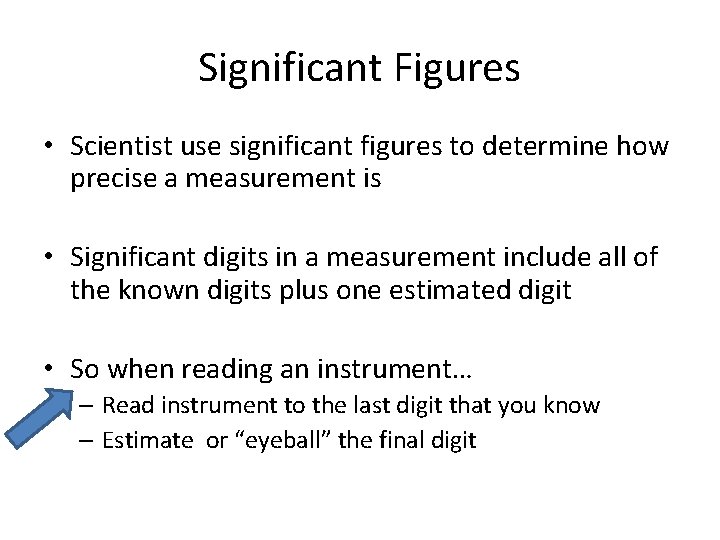
Significant Figures • Scientist use significant figures to determine how precise a measurement is • Significant digits in a measurement include all of the known digits plus one estimated digit • So when reading an instrument… – Read instrument to the last digit that you know – Estimate or “eyeball” the final digit

For example… • Look at the ruler below • Each line is 0. 1 cm • You can read that the arrow is on 13. 3 cm • However, using significant figures, you must estimate the next digit • That would give you 13. 30 cm

Let’s try this one • Look at the ruler below • • What can you read before you estimate? 12. 8 cm Now estimate the next digit… 12. 85 cm

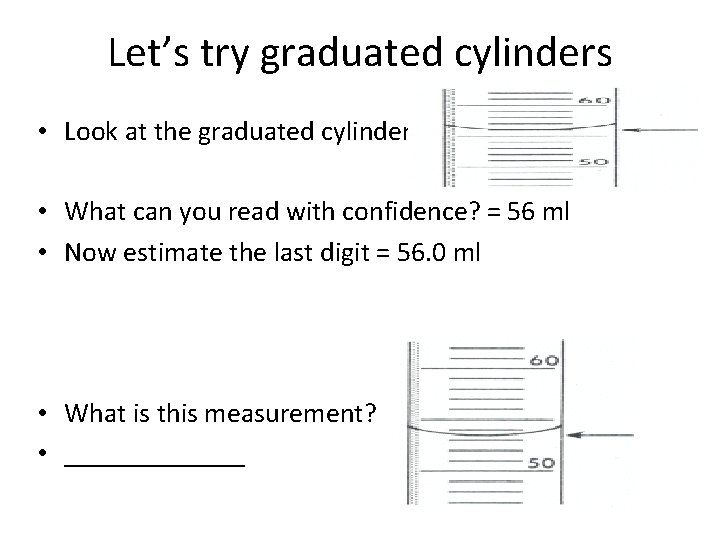
Let’s try graduated cylinders • Look at the graduated cylinder • What can you read with confidence? = 56 ml • Now estimate the last digit = 56. 0 ml • What is this measurement? • _______

Recognizing # Sig Figs in a Number • All non zero digits are ALWAYS significant • How many significant digits are in the following numbers? 274 ______Significant Figures 25. 632 ______Significant Digits 8. 987 ______Significant Figures

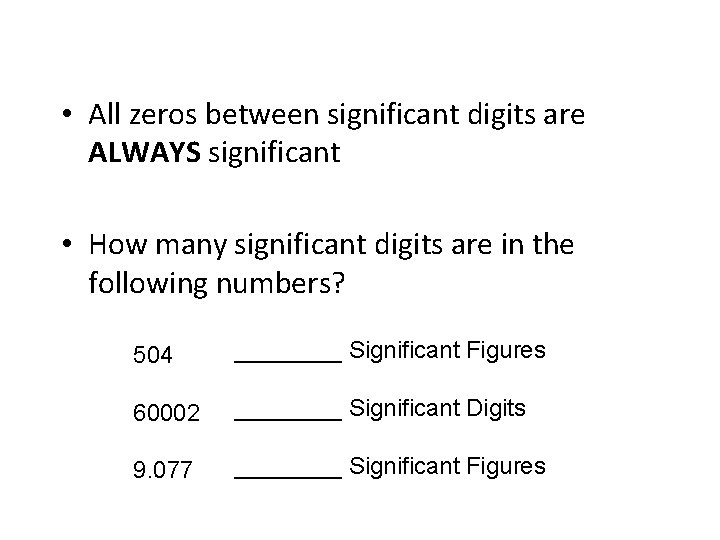
• All zeros between significant digits are ALWAYS significant • How many significant digits are in the following numbers? 504 ____ Significant Figures 60002 ____ Significant Digits 9. 077 ____ Significant Figures

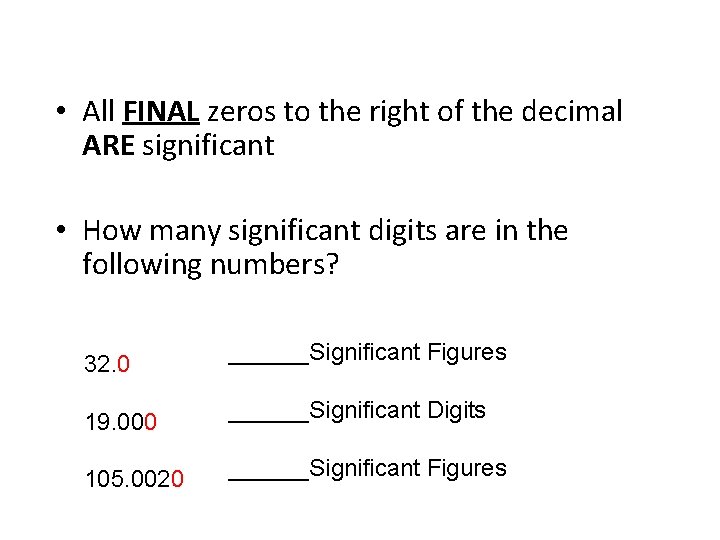
• All FINAL zeros to the right of the decimal ARE significant • How many significant digits are in the following numbers? 32. 0 ______Significant Figures 19. 000 ______Significant Digits 105. 0020 ______Significant Figures

All zeros that act as place holders are NOT significant Another way to say this is: zeros are only significant if they are between significant digits OR are the very final thing at the end of a decimal How many significant digits are in the following numbers? 0. 0002 6. 02 x 1023 100. 000 150000 800 _____Significant Digits _____Significant Digit

Numbers with no decimal are ambiguous. . . • Does 5000 ml mean exactly 5000? Maybe. . Maybe Not! • So 5000, 50, and 5 are all assumed to have 1 significant figure • If a writer means exactly 5000, he/she must write 5000. or 5. 000 x 103

• All counting numbers and constants have an infinite number of significant digits • For example: 1 hour = 60 minutes 12 inches = 1 foot 24 hours = 1 day

How many significant digits are in the following numbers? 0. 0073 100. 020 2500 7. 90 x 10 -3 670. 00001 18. 84 _______________ _______________

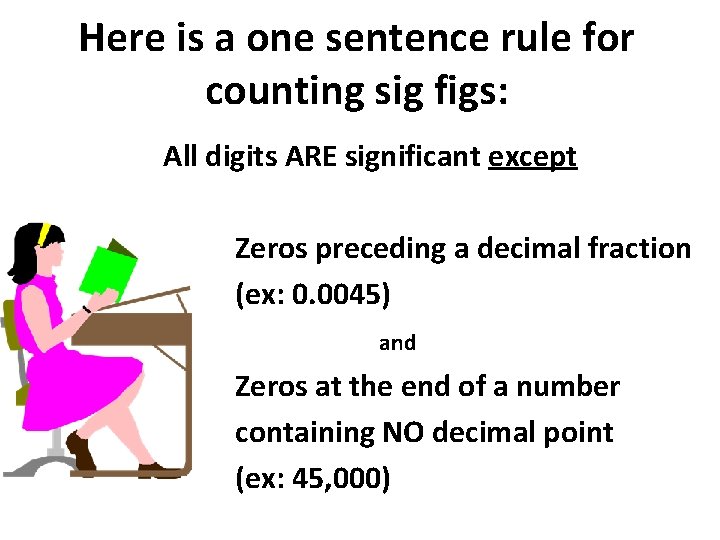
Here is a one sentence rule for counting sig figs: All digits ARE significant except Zeros preceding a decimal fraction (ex: 0. 0045) and Zeros at the end of a number containing NO decimal point (ex: 45, 000)

Calculations with Sig Figs • Adding or subtracting: – answer can have no more places after the decimal than the LEAST of the measured numbers. • Count # decimal places held – (nearest. 1? . 001? ) • Answer can be no more accurate than the LEAST accurate number that was used to calculate it.

For Example: 5. 50 grams + 8. 6 grams -------14. 1 grams 52. 09 ml - 49. 7 ml ------2. 39 ml --> 2. 4 ml

Calculations with Sig Figs • Multiplying or dividing: round result to least # of sig figs present in the factors – Answer can’t have more significant figures than the least reliable measurement. • COUNT significant figures in the factors

• 56. 78 cm x 2. 45 cm = 139. 111 cm 2 –Round to 3 sig figs = 139 cm 2 • 75. 8 cm x 9. 6 cm = ?

Now let’s do some math. . . (round answers to correct sig figs!) 5. 0033 g + 1. 55 g • answer: 6. 55 g Did you need to count sig figs? NO!

Try this one. . 4. 80 ml -. 0015 ml • answer: 4. 80 ml (one might say. 0015 is insignificant COMPARED TO 4. 80)

Now try these. . . 5. 0033 g / 5. 0 ml • answer: 1. 0 g/ml Did you have to count sig figs? • YES!

Here’s a tougher one. . . 3. 0 C/s x 60 s/min x 60 min/hr = • answer: 10800 C/hr rounds to 11000 C/hr Note: Standard conversion factors never limit sig. figures- instruments and equipment do.

Scientific Notation • Scientific notation is used to express very large or very small numbers • It consists of a number between 1 & 10 followed by x 10 to an exponent • Exponent can be determined by the number of decimal places you have to move to get only 1 number in front of the decimal

Large Numbers • If the number you start with is greater than 1, the exponent will be positive • Write the number 39923 in scientific notation • First move the decimal until 1 number is in front 3. 9923 • Now add x 10 • Now count the number of decimal places you moved (4) • Since the number you started with was greater than 1, the exponent will be positive • 3. 9923 x 10 4

Small Numbers • If the number you start with is less than 1, the exponent will be negative • Write the number 0. 0052 in scientific notation • First move the decimal until 1 number is in front = 5. 2 • Now add x 10 • Now count the number of decimal places moved (3) • Since the number you started with was less than 1, the exponent will be negative • 5. 2 x 10 -3

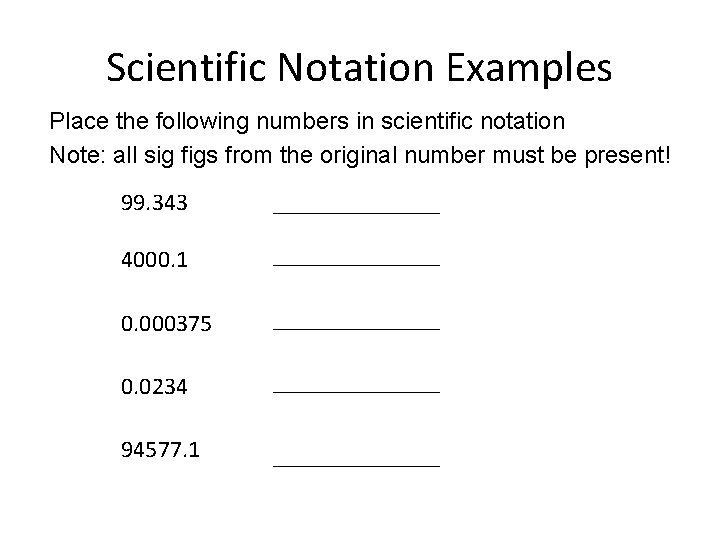
Scientific Notation Examples Place the following numbers in scientific notation Note: all sig figs from the original number must be present! 99. 343 _______ 4000. 1 _______ 0. 000375 _______ 0. 0234 _______ 94577. 1 _______

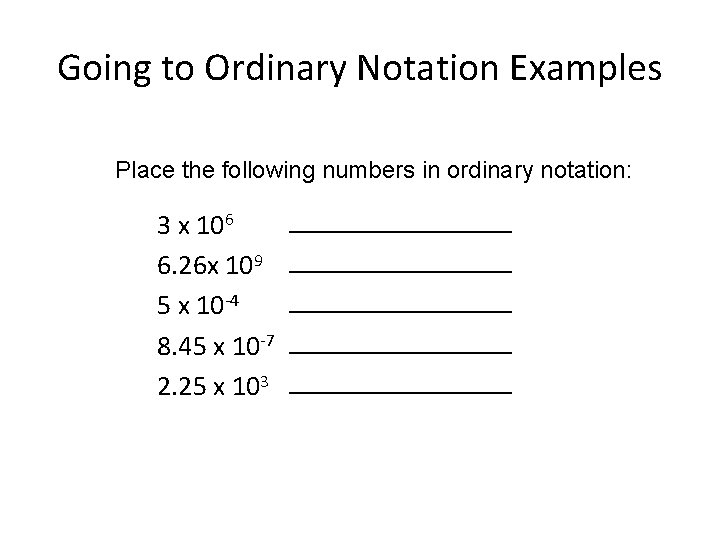
Going to Ordinary Notation Examples Place the following numbers in ordinary notation: 3 x 106 6. 26 x 109 5 x 10 -4 8. 45 x 10 -7 2. 25 x 103 ________________ ________