Signal Processing in Immune Cell Chemotaxis Matt Onsum










- Slides: 36

Signal Processing in Immune Cell Chemotaxis Matt Onsum UC Berkeley June 15, 2005

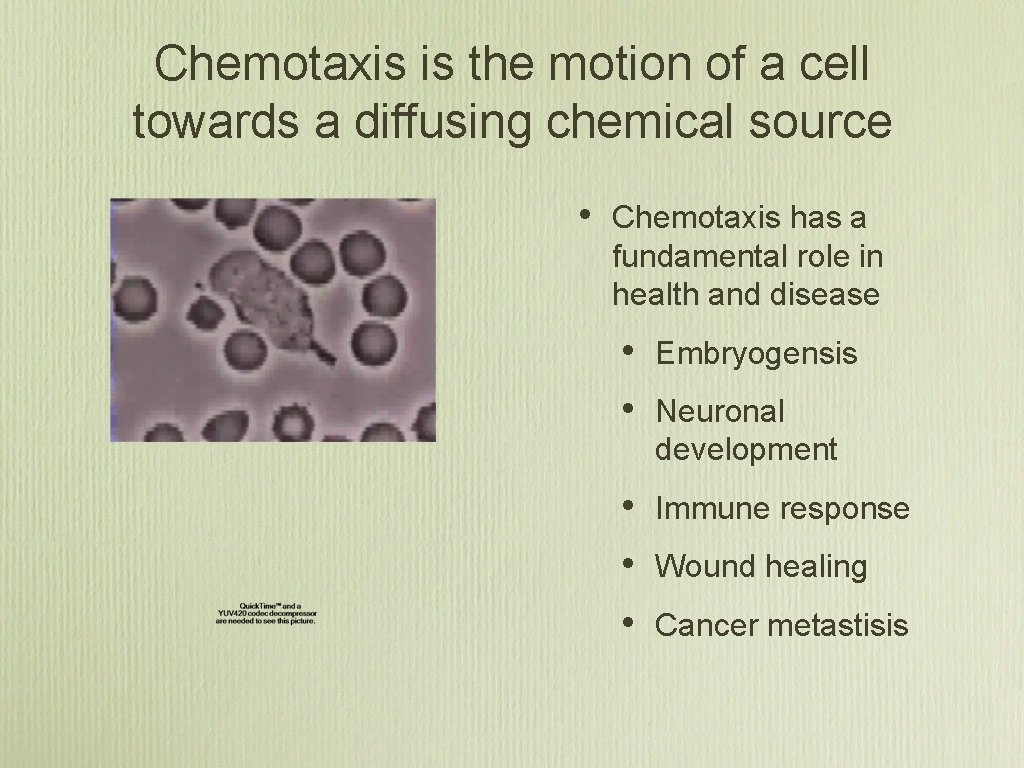
Chemotaxis is the motion of a cell towards a diffusing chemical source • Chemotaxis has a fundamental role in health and disease • • Embryogensis • • • Immune response Neuronal development Wound healing Cancer metastisis

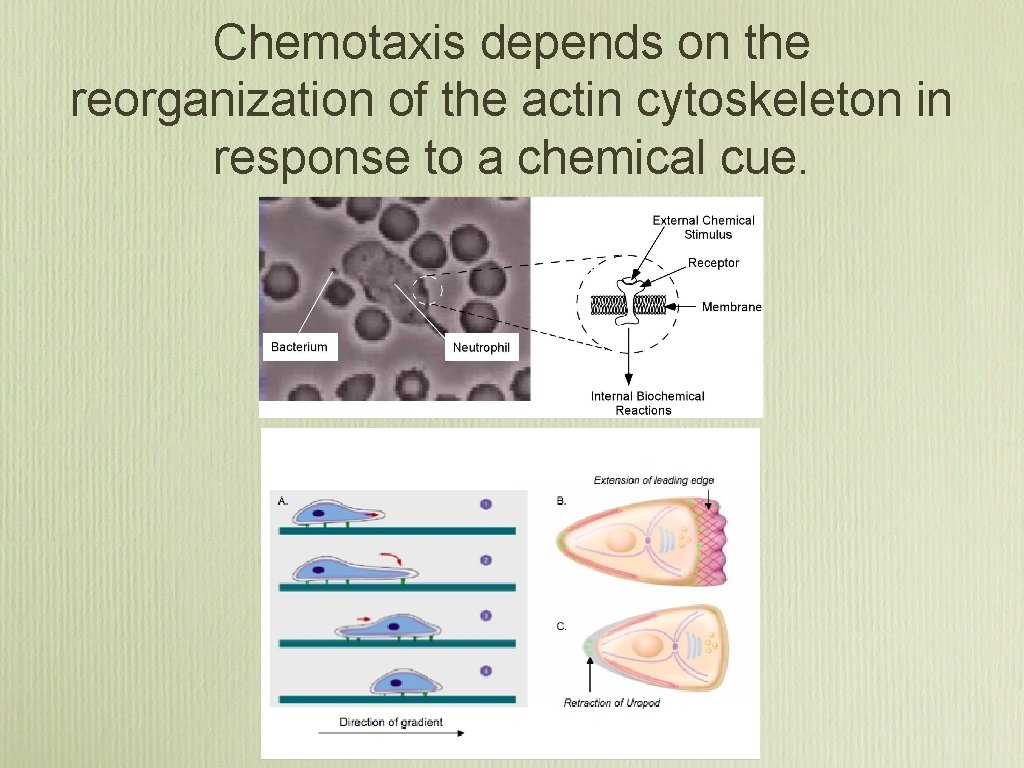
Chemotaxis depends on the reorganization of the actin cytoskeleton in response to a chemical cue.

How does the cell convert a shallow gradient into a localized response of the cytoskeleton? • Some early observations (Zigmond 1977): • Neutrophils respond to a 2% difference of chemoattractant across their length • Sensitive Front, insensitive back

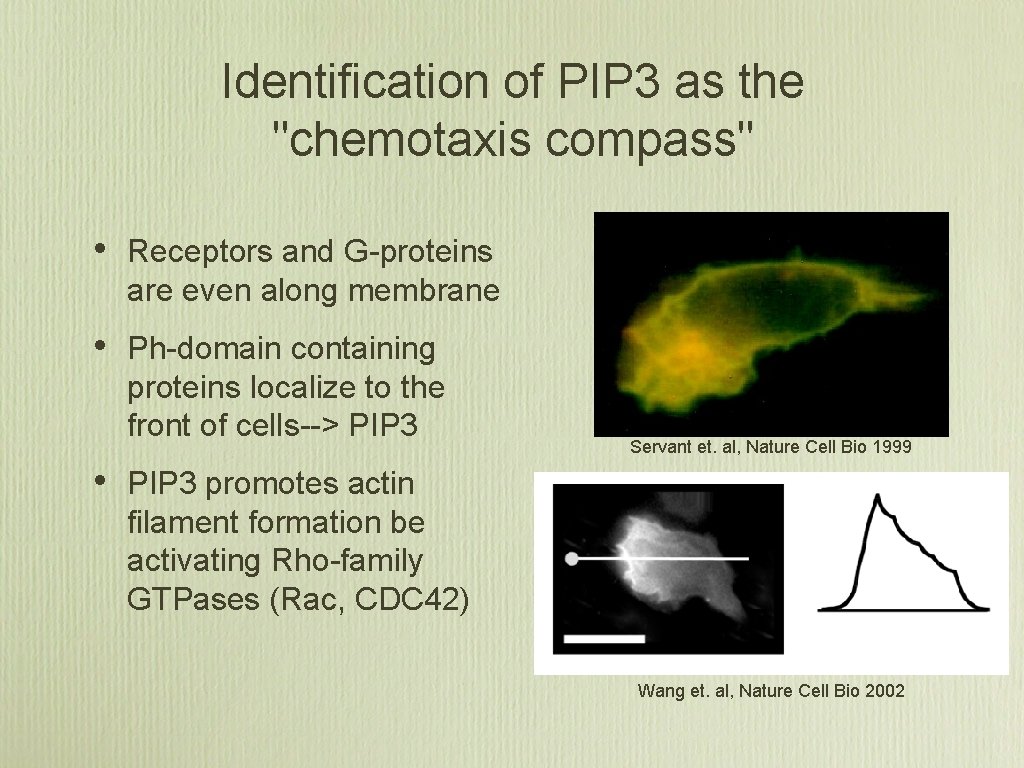
Identification of PIP 3 as the "chemotaxis compass" • Receptors and G-proteins are even along membrane • Ph-domain containing proteins localize to the front of cells--> PIP 3 • Servant et. al, Nature Cell Bio 1999 PIP 3 promotes actin filament formation be activating Rho-family GTPases (Rac, CDC 42) Wang et. al, Nature Cell Bio 2002

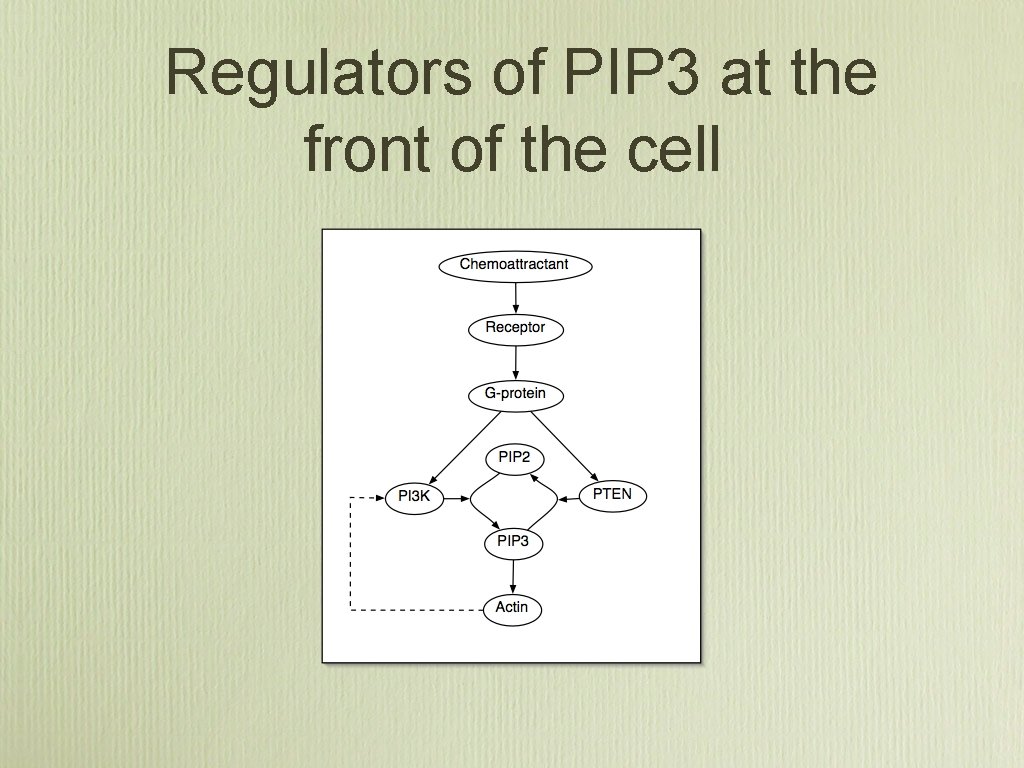
Regulators of PIP 3 at the front of the cell

Our aim was to quantify properties of this network • What is the amplification of network? • How does an asymetric receptor distribtion affect this amplification? • Does the amplfication machinary cause "selflocking" behavior? Servant et. al, Mol. Bio. Cell 1999

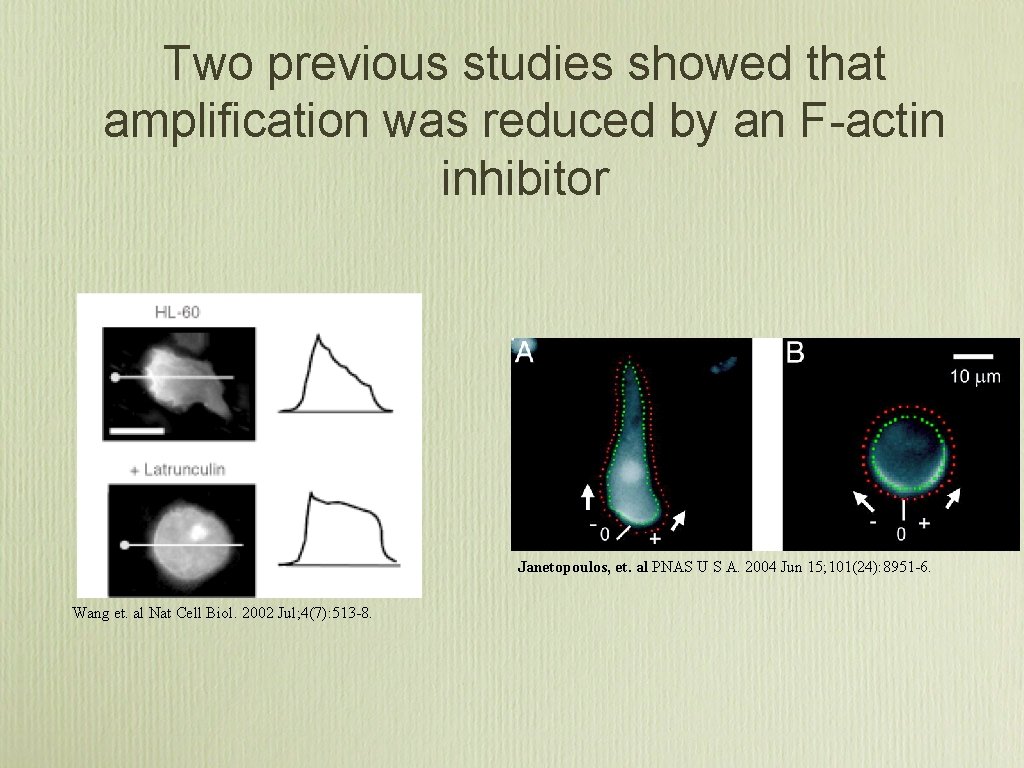
Two previous studies showed that amplification was reduced by an F-actin inhibitor Janetopoulos, et. al PNAS U S A. 2004 Jun 15; 101(24): 8951 -6. Wang et. al Nat Cell Biol. 2002 Jul; 4(7): 513 -8.

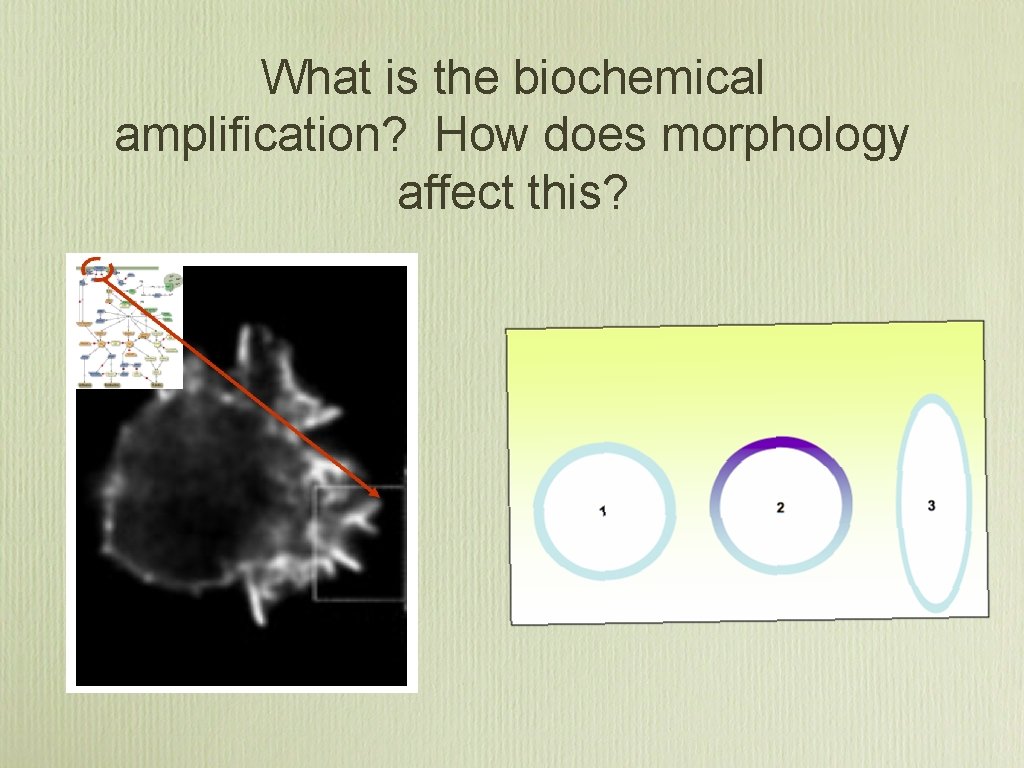
What is the biochemical amplification? How does morphology affect this?

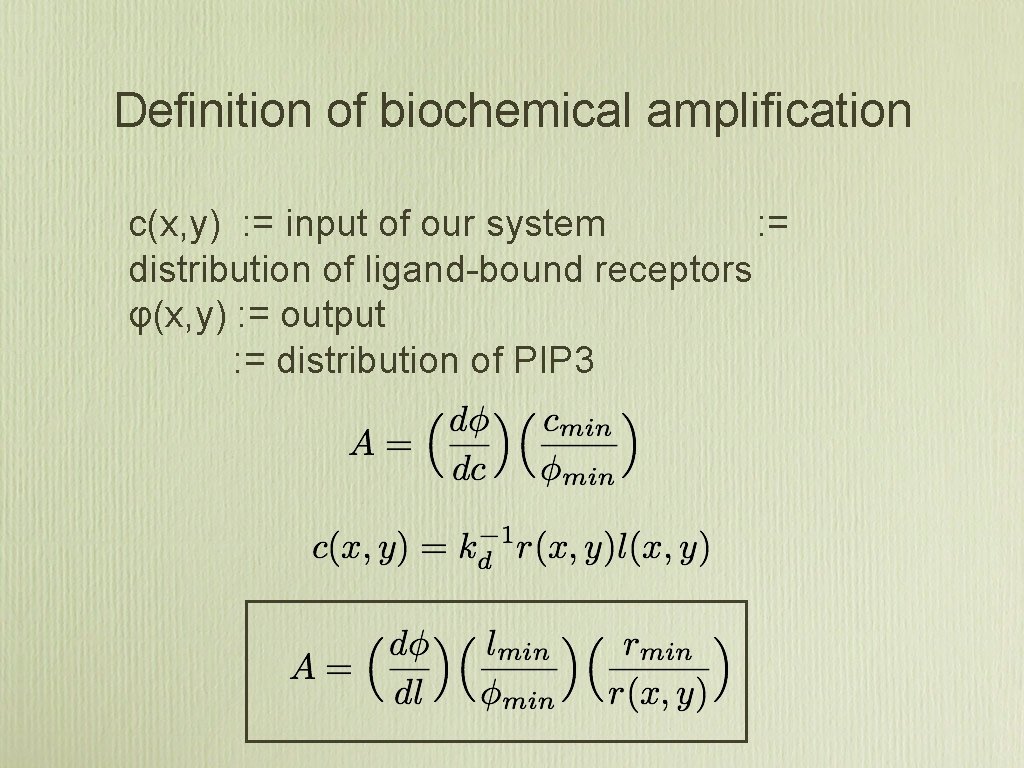
Definition of biochemical amplification c(x, y) : = input of our system : = distribution of ligand-bound receptors φ(x, y) : = output : = distribution of PIP 3

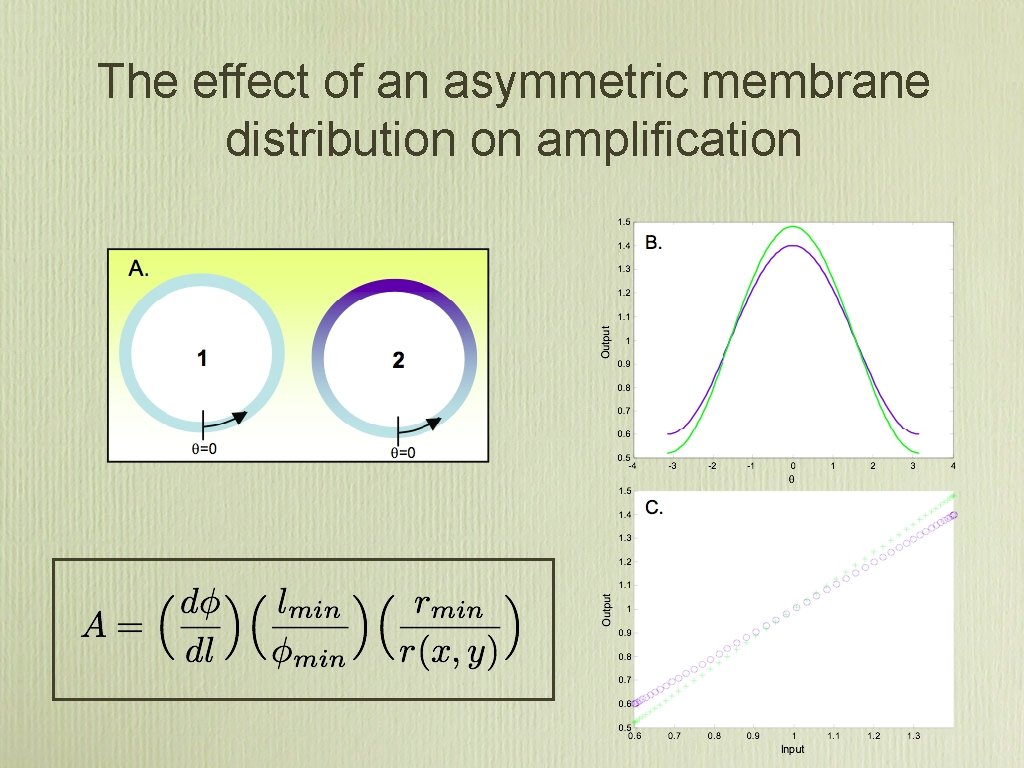
The effect of an asymmetric membrane distribution on amplification

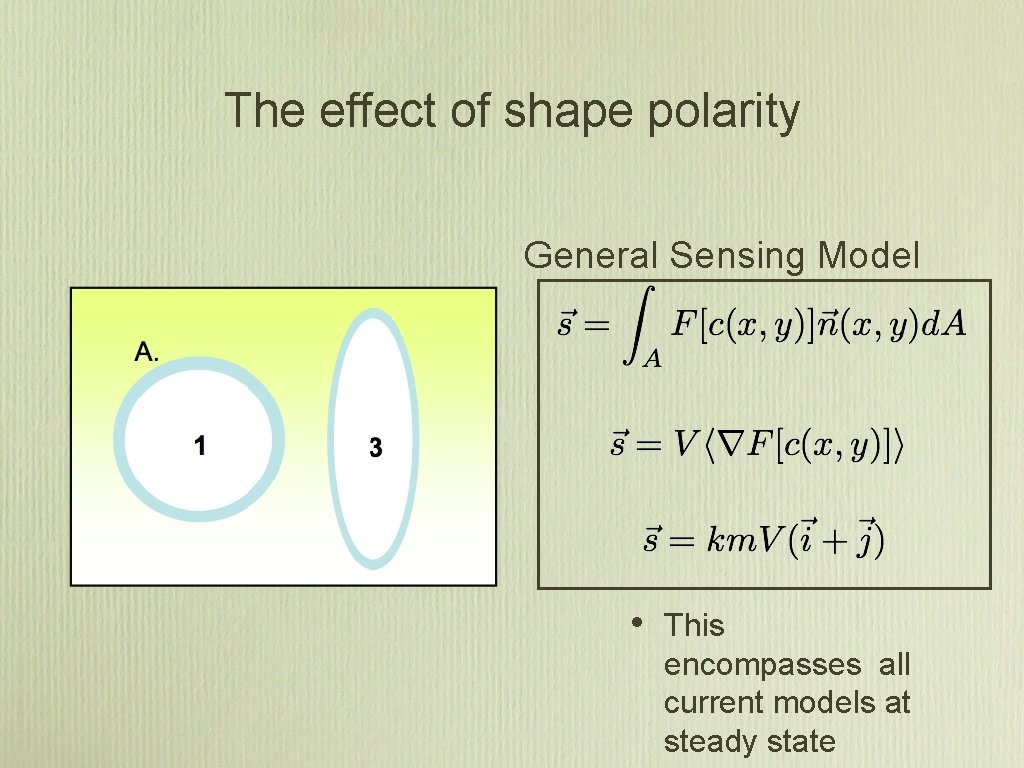
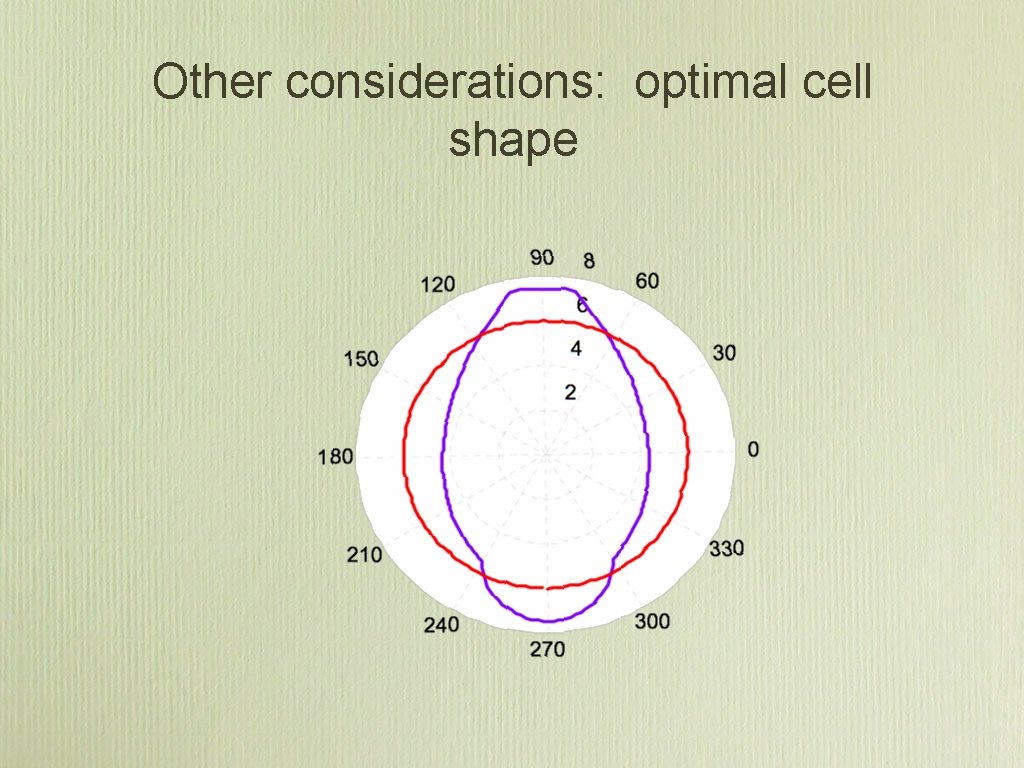
The effect of shape polarity General Sensing Model • This encompasses all current models at steady state

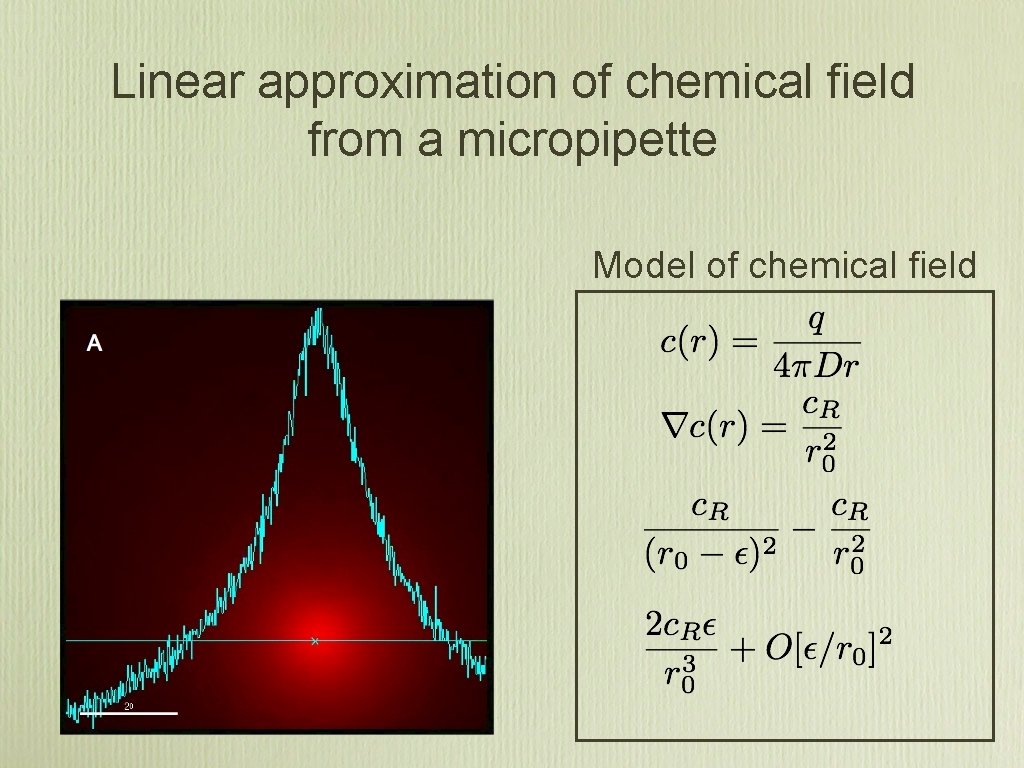
Linear approximation of chemical field from a micropipette Model of chemical field

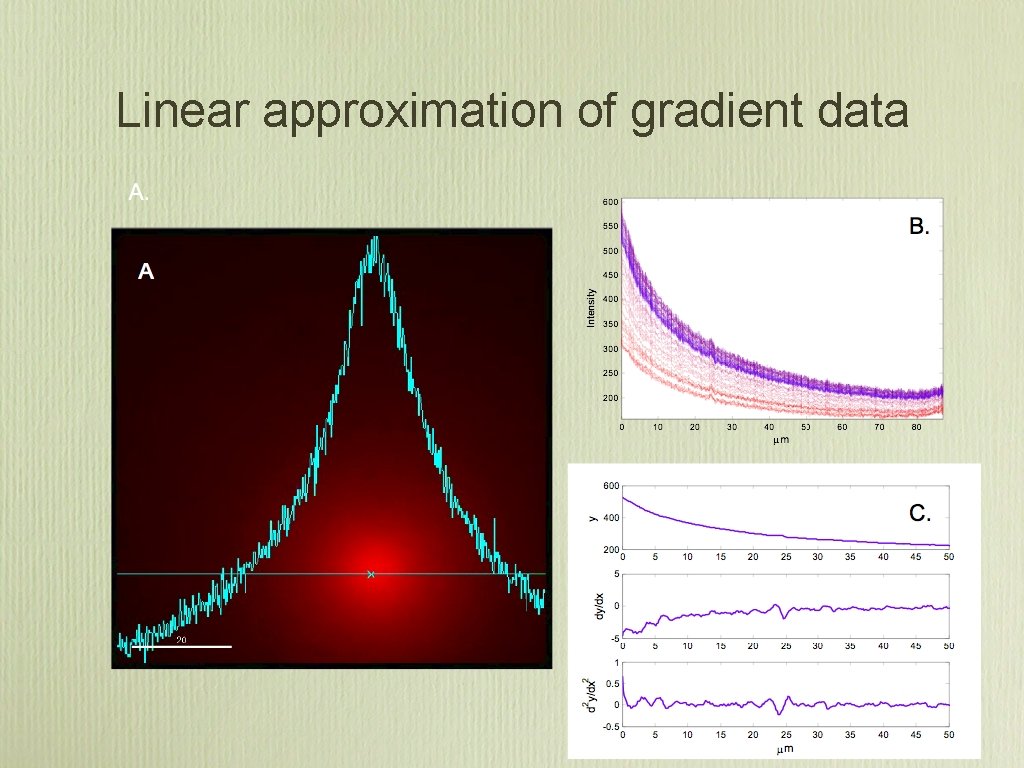
Linear approximation of gradient data

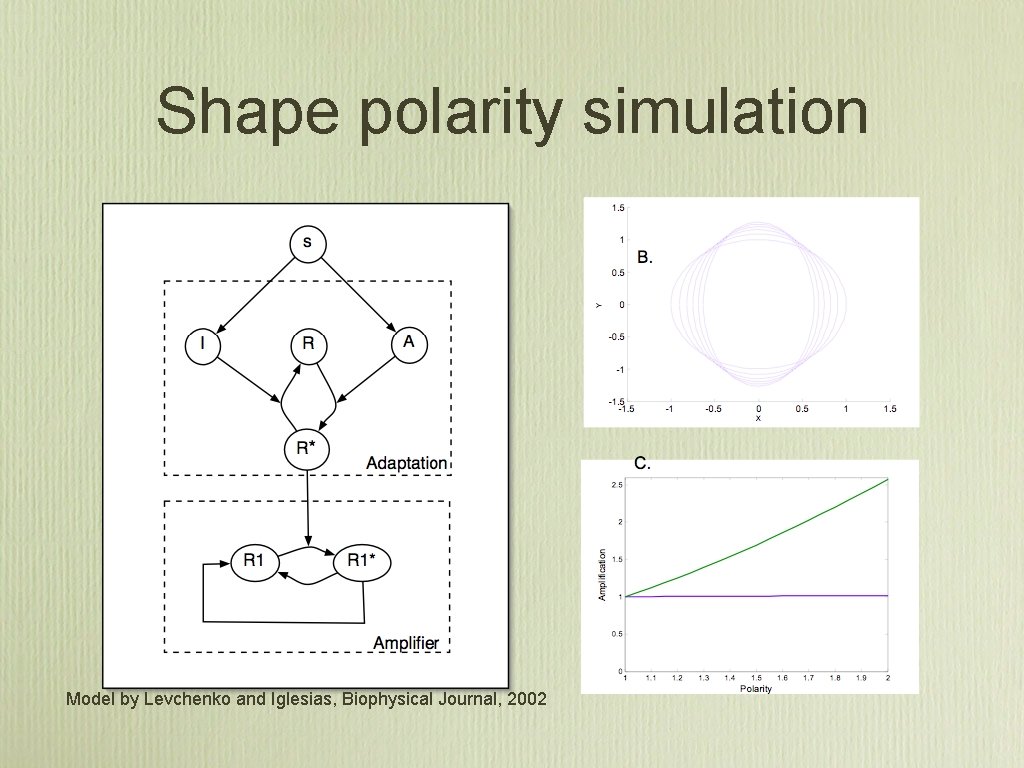
Shape polarity simulation Model by Levchenko and Iglesias, Biophysical Journal, 2002

Other considerations: optimal cell shape

Summary thus far. . . • An asymmetric membrane/receptor distribution will affect measures of biochemical amplification • Shape polarity will affect amplification if the underlying biochemical circuit behaves as a nonlinear amplifier

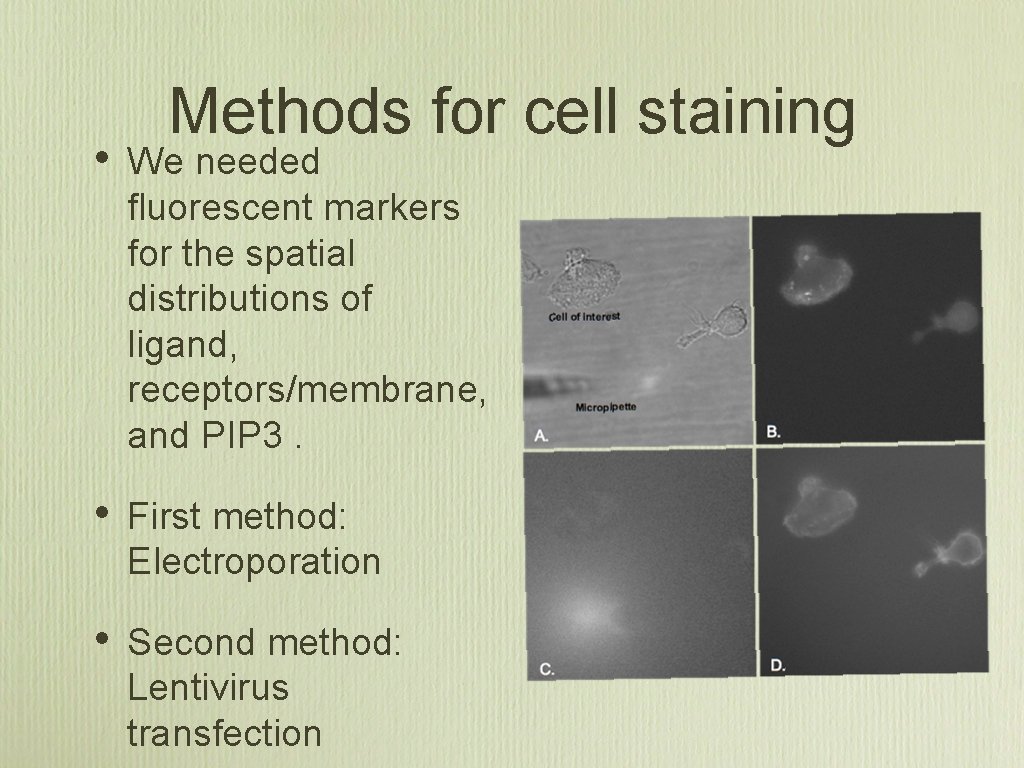
Methods for cell staining • We needed fluorescent markers for the spatial distributions of ligand, receptors/membrane, and PIP 3. • First method: Electroporation • Second method: Lentivirus transfection

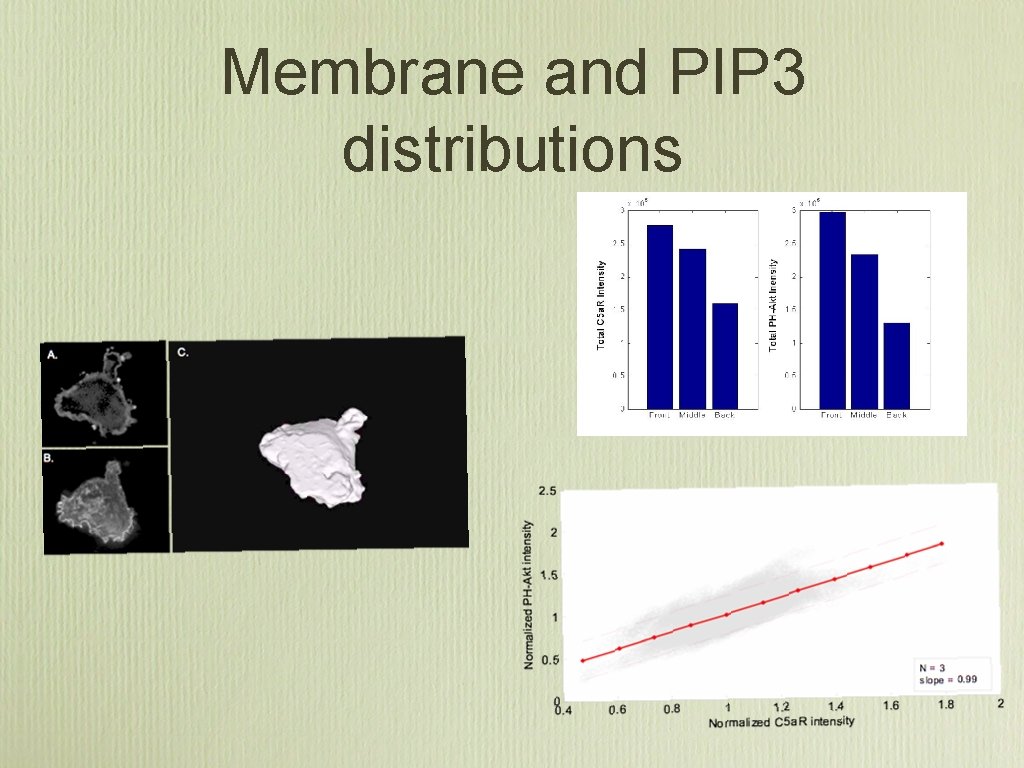
Membrane and PIP 3 distributions

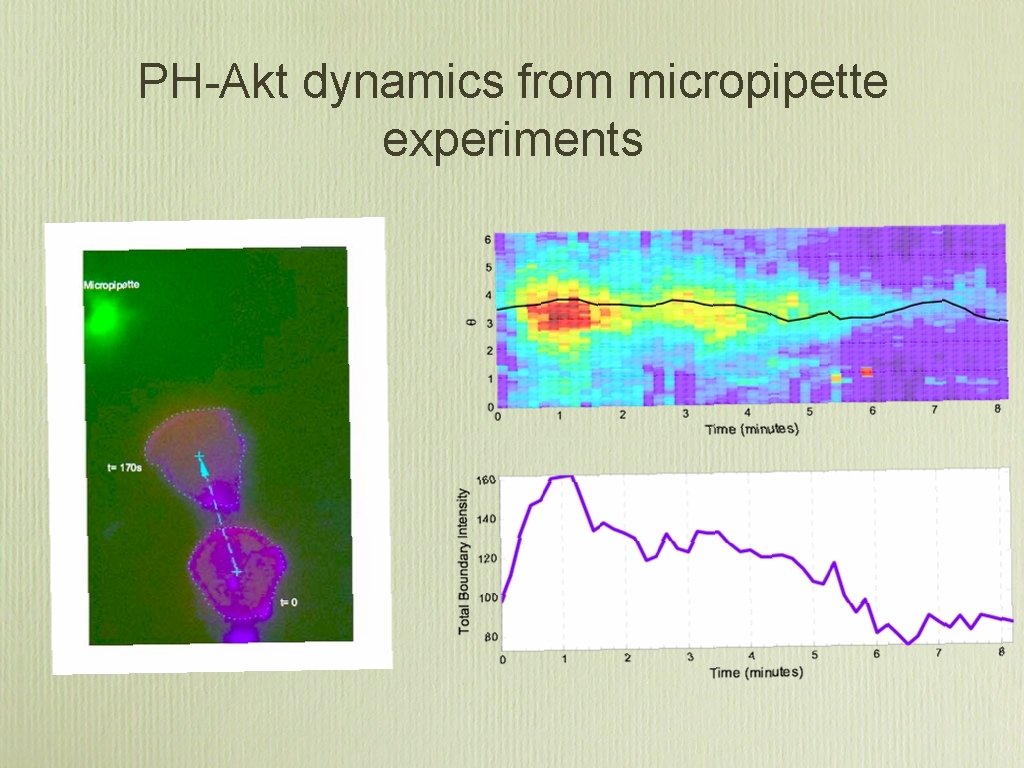
Example of micropipette experiment to measure amplification

PH-Akt dynamics from micropipette experiments

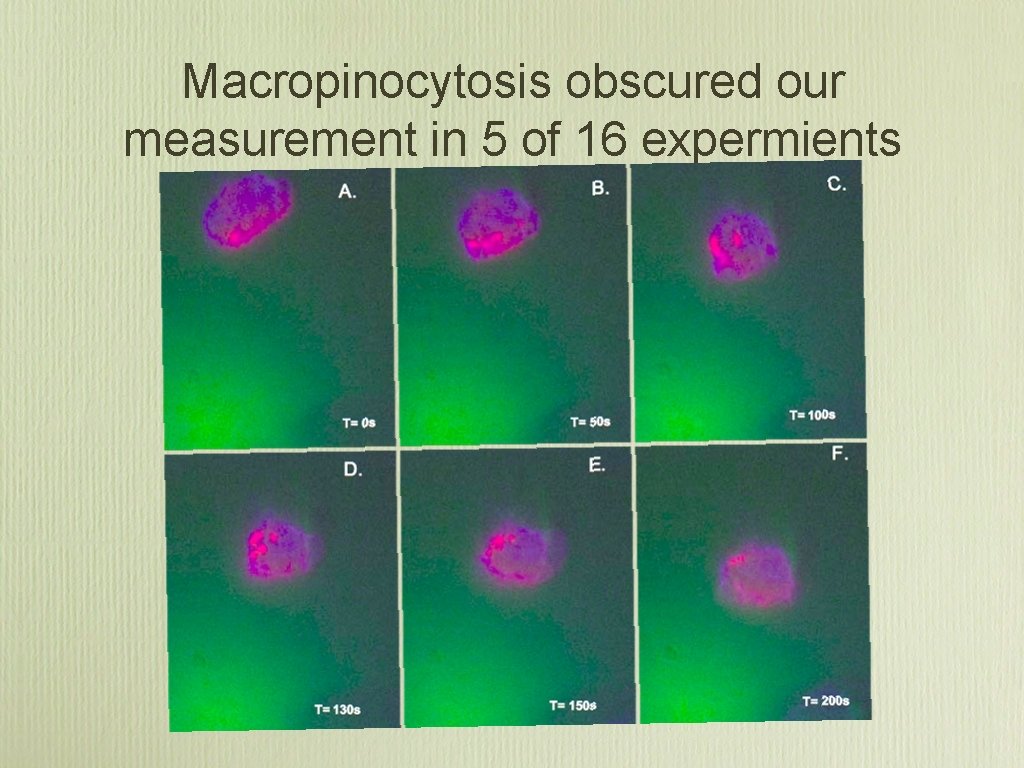
Macropinocytosis obscured our measurement in 5 of 16 expermients

Amplification Results • Membrane Normalized Amplification = 3. 25 ± 2. 0 • Unnormalized Amplification = 5. 35 ± 2. 5 • Signification at the P = 0. 002 level from the paired T-test

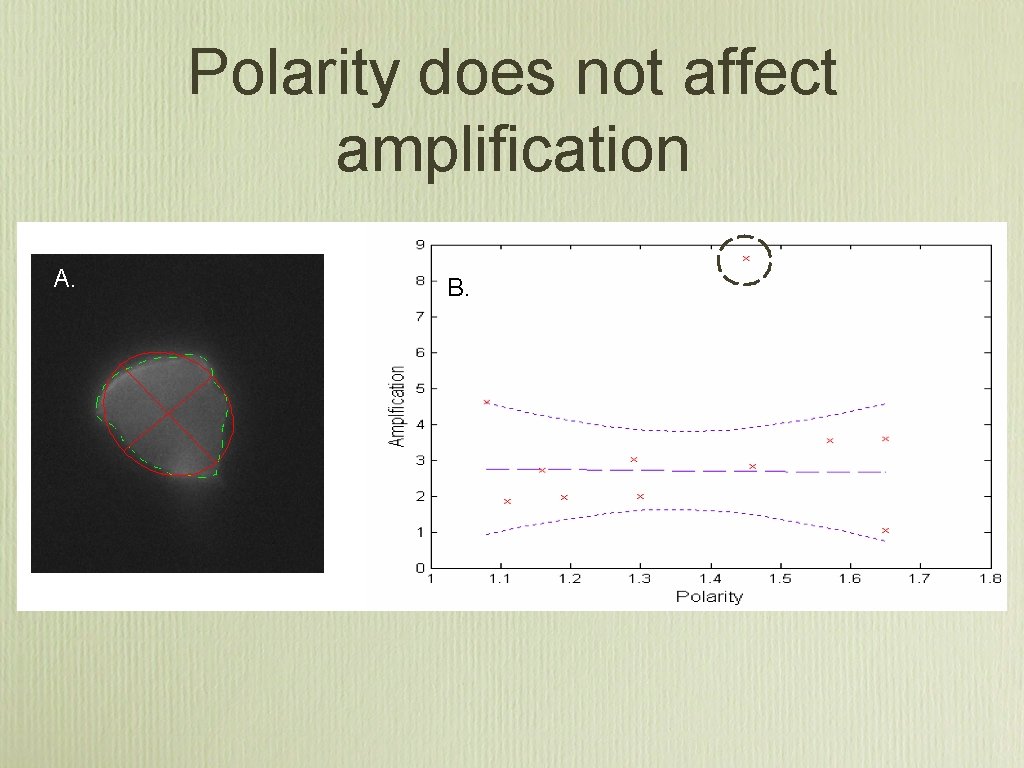
Polarity does not affect amplification

Results summary • There are more receptors at the leading edge of a chemotaxing cell then the back (25% ± 10), and this strongly correlates with the PIP 3 distribution • The biochemical amplification is 3. 25 ± 2. 0 This is 49% less then it would be if the receptor distribution was assumed constant. • There is no association between shape polarity and amplification, which implies that the underlying biochemical network behaves as a linear amplifier.

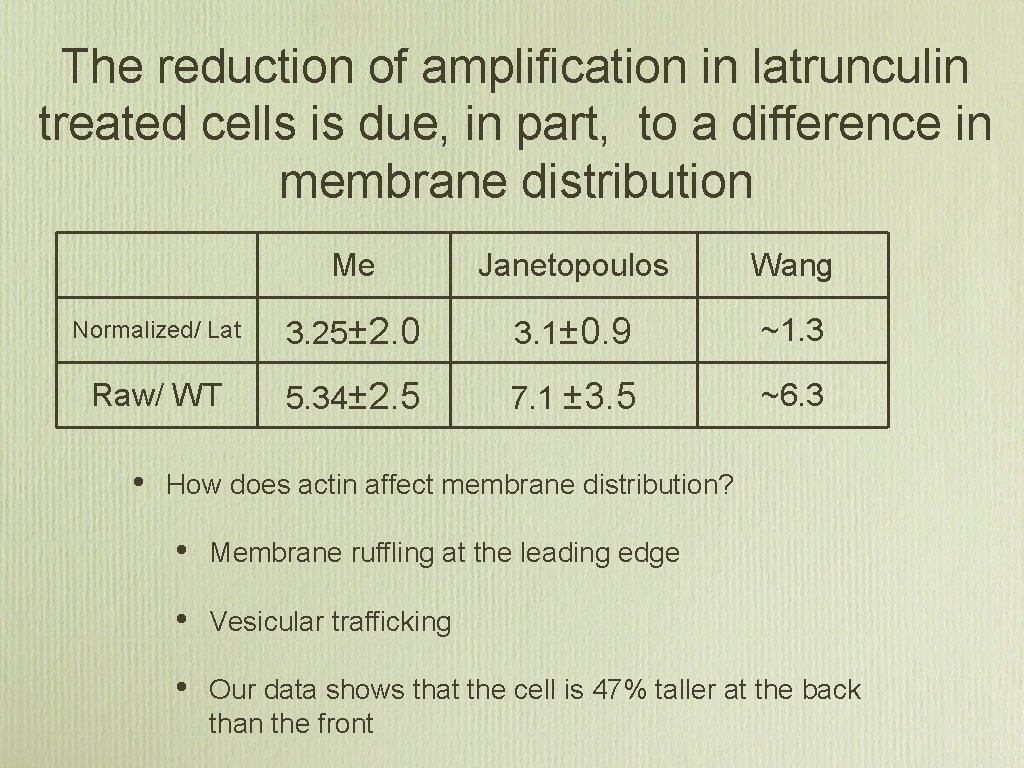
The reduction of amplification in latrunculin treated cells is due, in part, to a difference in membrane distribution Me Janetopoulos Wang Normalized/ Lat 3. 25± 2. 0 3. 1± 0. 9 ~1. 3 Raw/ WT 5. 34± 2. 5 7. 1 ± 3. 5 ~6. 3 • How does actin affect membrane distribution? • Membrane ruffling at the leading edge • Vesicular trafficking • Our data shows that the cell is 47% taller at the back than the front

Additional results. . . • The time response of the PIP 3 network to 90˚ changes in gradient direction is 20. 0 ± 1. 3 seconds. (There is a mechanism to prevent self-locking) • The speed of a chemotaxing HL 60 is constant = [2. 5, 4]microns/minute

Brief Collaborative Control review • Control is based on multiple controllers controlling a single robots • Sensor fusion • Multiple human operators • Subsumption of multiple sources • Unlike most algorithms for autonomous robots, this does not require a potential map • It has been shown in simulation that this strategy is robust to faulty and malignant sources.

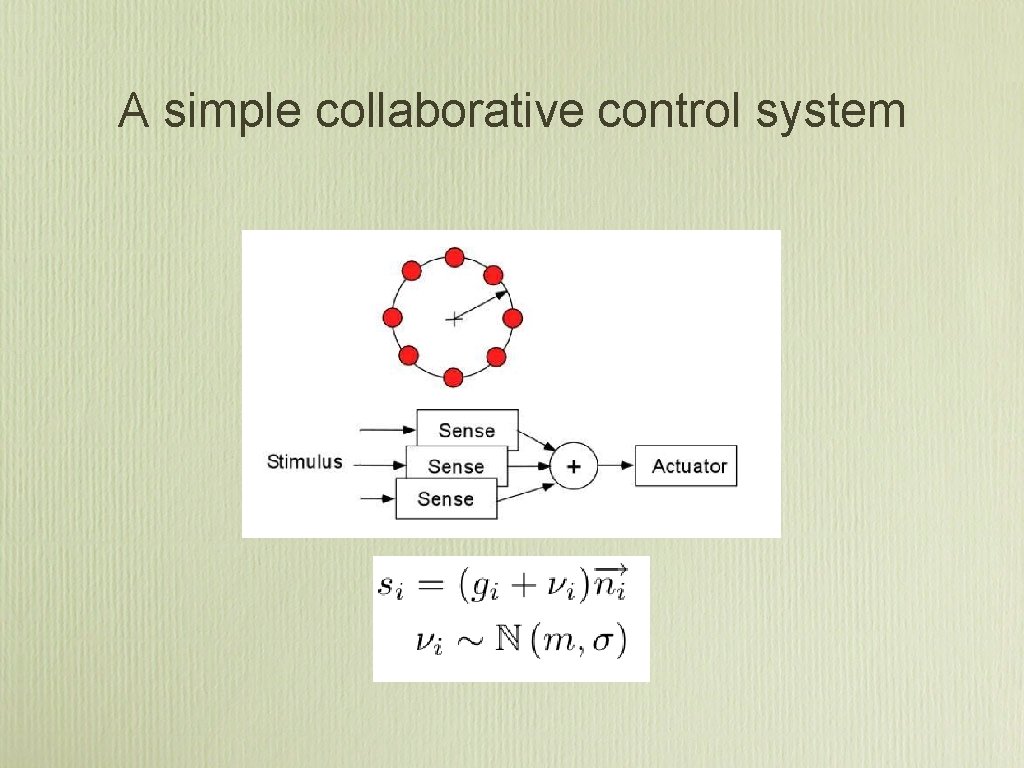
A simple collaborative control system

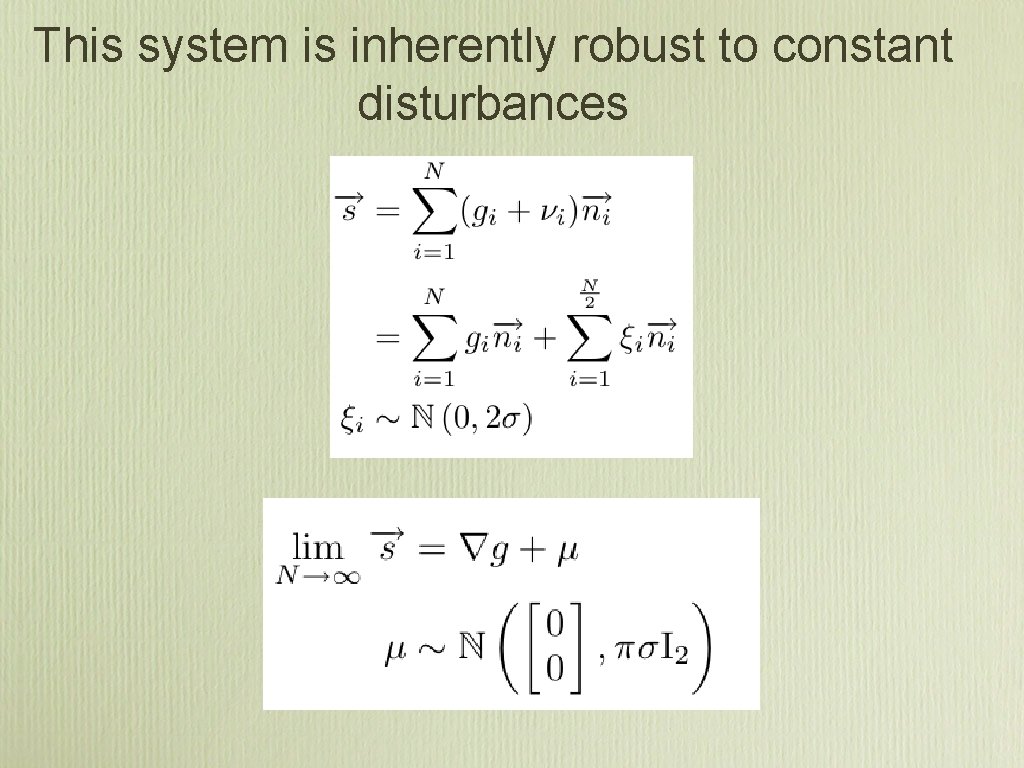
This system is inherently robust to constant disturbances

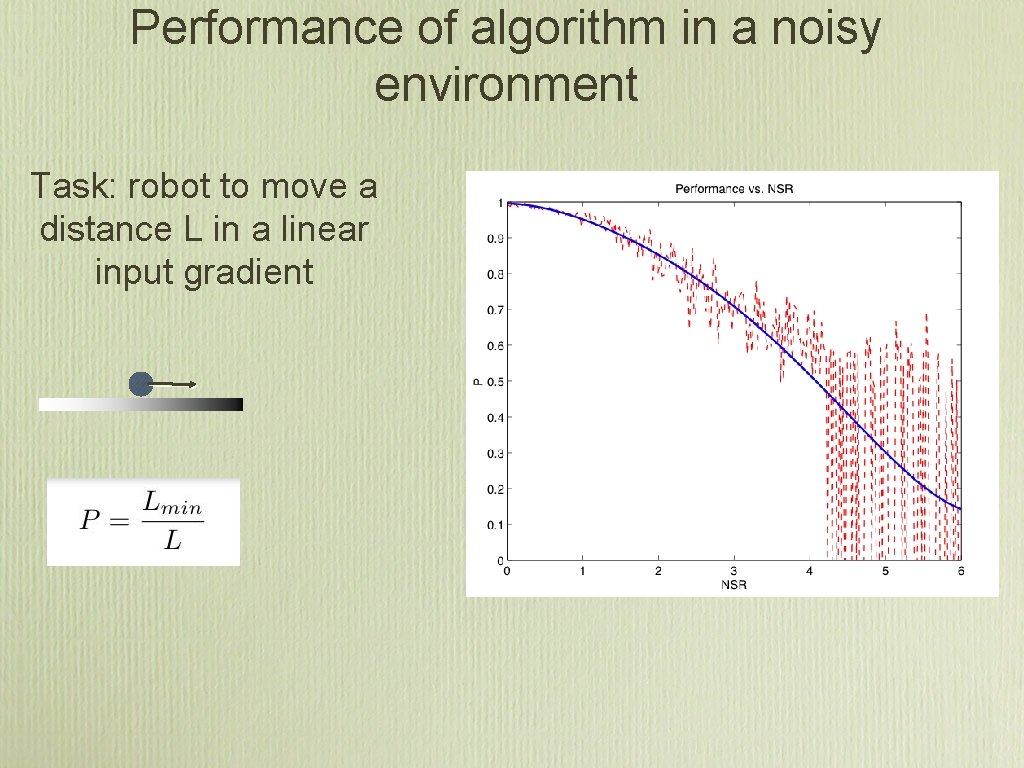
Performance of algorithm in a noisy environment Task: robot to move a distance L in a linear input gradient

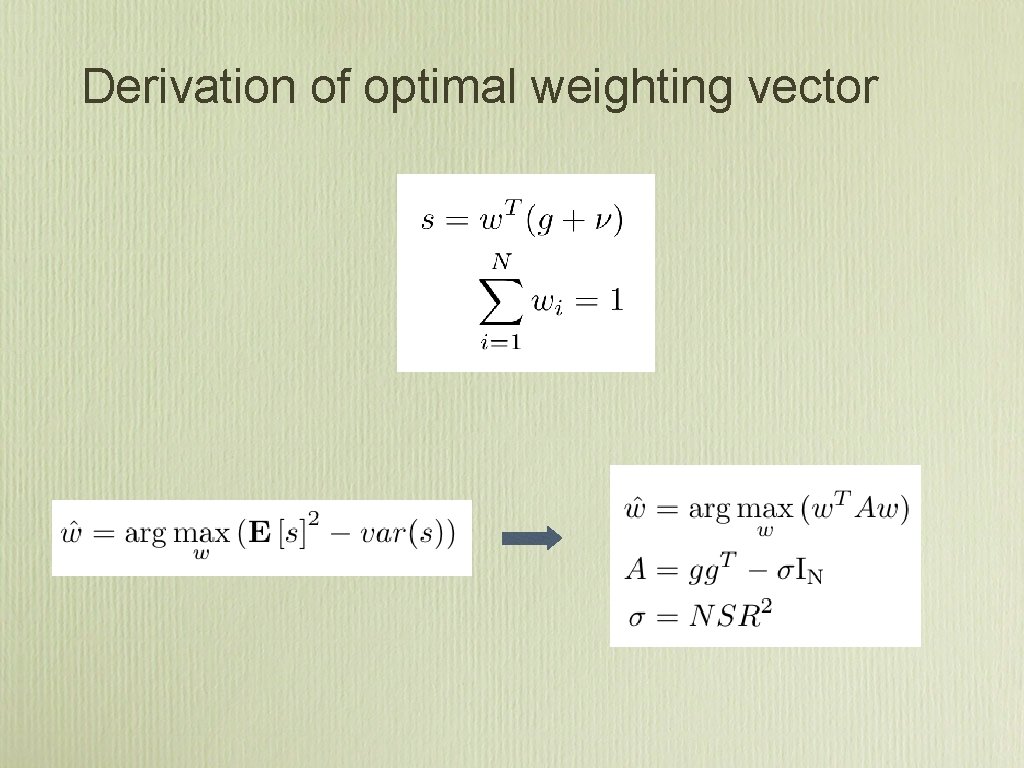
Derivation of optimal weighting vector

Polarity of w vs. NSR

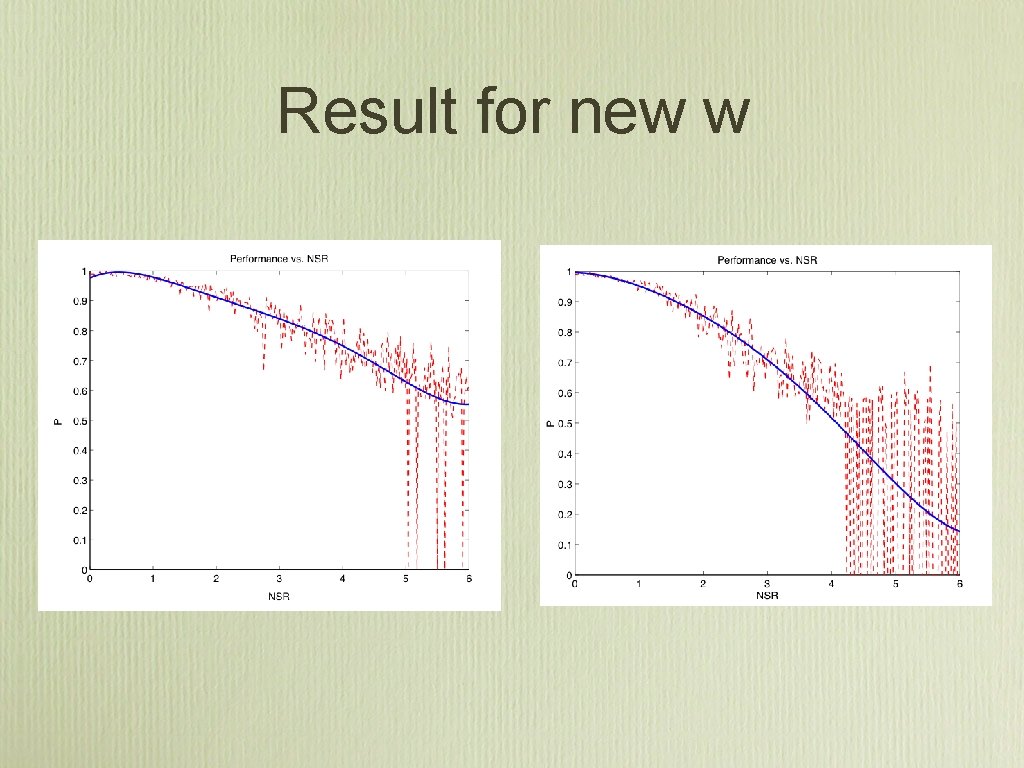
Result for new w

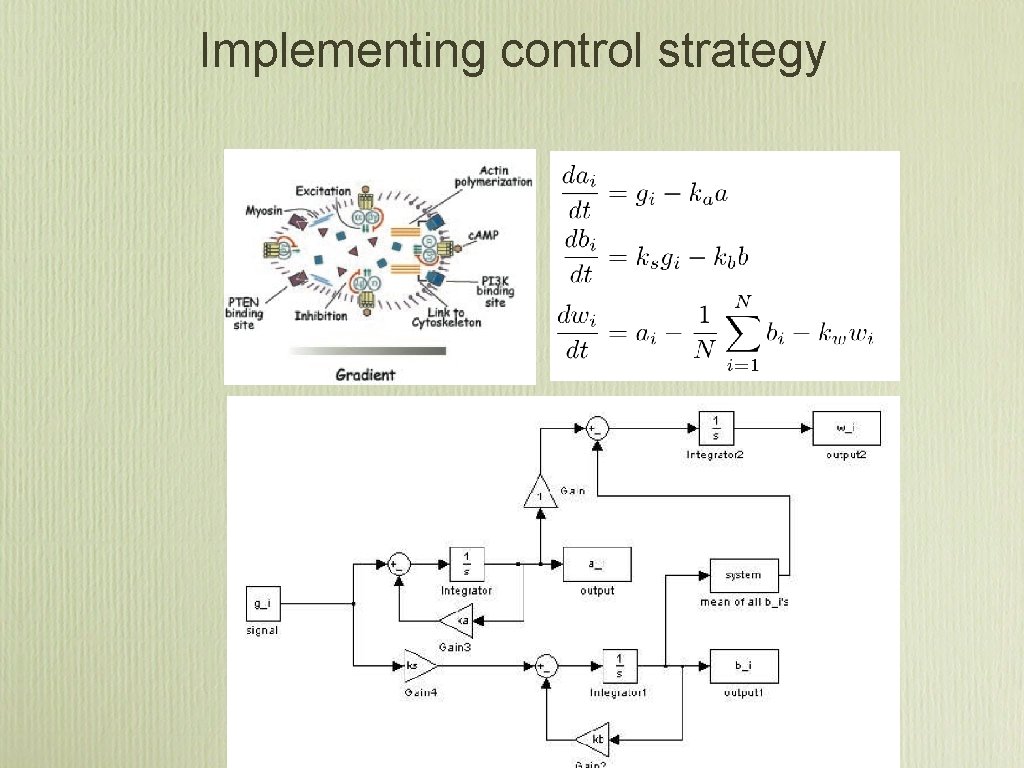
Implementing control strategy

Acknowledgments • Arkin Lab, UC Berkeley • Bourne Lab, UCSF - Kit Wong & Paul Herzmark • BCCI • NIH