SIGN GUIDELINE DIAGNOSIS AND MANAGEMENT OF DELIRIUM Ailsa

















- Slides: 20

SIGN GUIDELINE: DIAGNOSIS AND MANAGEMENT OF DELIRIUM Ailsa Stein, Programme Manager, SIGN

Scottish Intercollegiate Guidelines Network • Public funding but professional ownership • evidence-based clinical guidelines produced using transparent methodology • internationally validated methodology.

Delirium guideline development group Consultant Physicians Consultant Psychiatrists Consultant in Geriatricians General Practitioner Pharmacist A & E Consultant SIGN Health economist Nurse Practitioner Consultant in Critical Care and Anaesthesia Lay representatives Anaesthetist

Patient/carer issues identified previously by Healthcare Improvement Scotland in relation to delirium • Value of family members – skills and experience in detecting early warning signs • Suitable approach from staff • Education and training for staff – early warning signs • Role of NHS 24 in getting expert knowledge from carers • Opportunity to discuss dreams or nightmares • Support for carers • Being kept in the loop - updates for family members • Staff could suggest to families keeping a diary

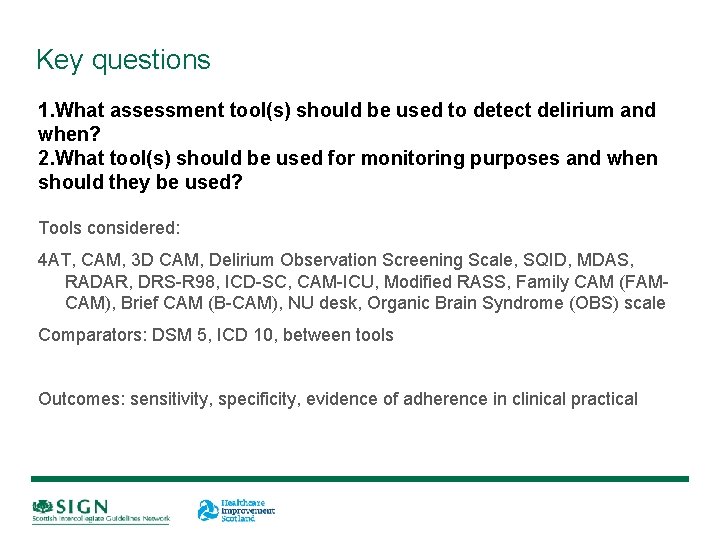
Key questions 1. What assessment tool(s) should be used to detect delirium and when? 2. What tool(s) should be used for monitoring purposes and when should they be used? 3. What (other) investigations are useful when assessing a patient for delirium? 4. What risk reduction strategies for patients at risk of delirium are effective? 5. What are the most effective non-pharmacological strategies for managing patients with delirium? 6. What are the most effective pharmacological strategies for managing patients with delirium? 7. What follow-up care should patients receive after experiencing delirium?

Key questions • Adults over 18 years at risk of, or experiencing, delirium • Home, long-term care, hospital, hospice • Excludes: delirium secondary to alcohol, patients with delirium tremens, paediatric delirium • Comborbidities considered: dementia, depression, frailty, head injury, learning disability, Parkinson’s disease, stroke

Key questions 1. What assessment tool(s) should be used to detect delirium and when? 2. What tool(s) should be used for monitoring purposes and when should they be used? Tools considered: 4 AT, CAM, 3 D CAM, Delirium Observation Screening Scale, SQID, MDAS, RADAR, DRS-R 98, ICD-SC, CAM-ICU, Modified RASS, Family CAM (FAMCAM), Brief CAM (B-CAM), NU desk, Organic Brain Syndrome (OBS) scale Comparators: DSM 5, ICD 10, between tools Outcomes: sensitivity, specificity, evidence of adherence in clinical practical

Key questions 3. What (other) investigations are useful when assessing a patient for delirium? • imaging (CT or MRI scans) • lumbar puncture • electroencephalogram (EEG) • testing for antibodies for autoimmune encephalitis • toxicology screening Comparison: usual care Outcomes: sensitivity, specificity, cost effectiveness

Key questions 4. What risk reduction strategies for patients at risk of delirium are effective? Non-pharmacological: Pharmacological: • hydration • medication reconciliation • catheterization avoidance • pain relief • sensory impairment • antipsychotics and benzodiazepines • constipation • sedation for night-time sleep • sleep hygiene and promotion • falls prevention and mobility • providing means of communication • impact of ward moves • environmental factors • proactive screening of delirium and pre-existing cognitive impairment including dementia

Key questions 5. What are the most effective non-pharmacological strategies for managing patients with delirium? • staff behavioural adaptations (calm manner, one-to-one nursing, cognitive stimulation, reassurance, reorientation, distraction/de-escalation techniques) • environmental adaptations (single room, lighting, clear signage for orientation, familiar objects, family input, minimise bed moves, address sensory impairment, OT, sleep promotion, facilitating mobility) • address specific causes of stress (pain, hunger, feeling hot or cold, thirsty, urinary retention, specific fears, not understanding what is happening, hallucinations, delusions, aggression, agitation, and searching)

Key questions 6. What are the most effective pharmacological strategies for managing patients with delirium? • antipsychotics • benzodiazepines • acetylcholinesterase inhibitors • melatonin • antidepressants • dexmedetomidine • clonidine • propanolol • withdrawal of culprit drugs

Key questions 7. What follow-up care should patients receive after experiencing delirium? Screening for: • dementia • functional psychiatry disorders – PTSD, depression

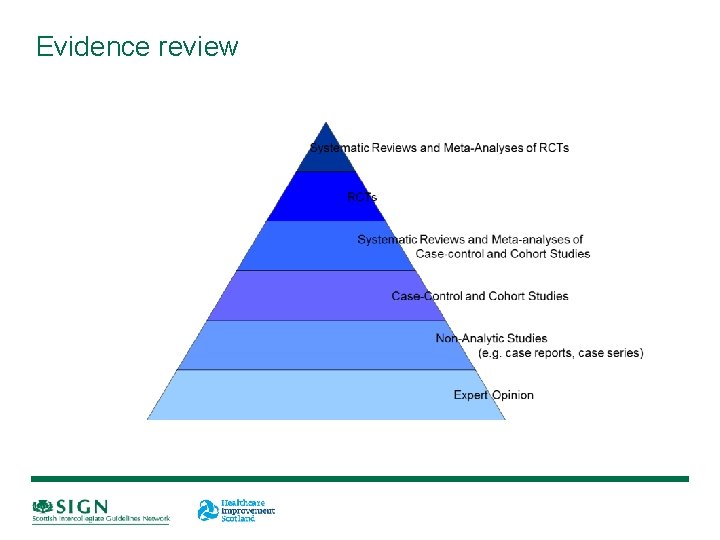
Evidence review

Forming recommendations Help clinicians with decisionmaking Systematically summarise the evidence base Consider the quality of the evidence base Balance the benefits and harms Create evidence-based, implementable recommendations Consider the realities of healthcare delivery

Forming recommendations Quality of evidence • quantity of evidence Applicability • benefits • consistency of results • harms • relevance to the target • impact on patients (patient population preferences) • feasibility (eg cost, service • any publication bias provision)

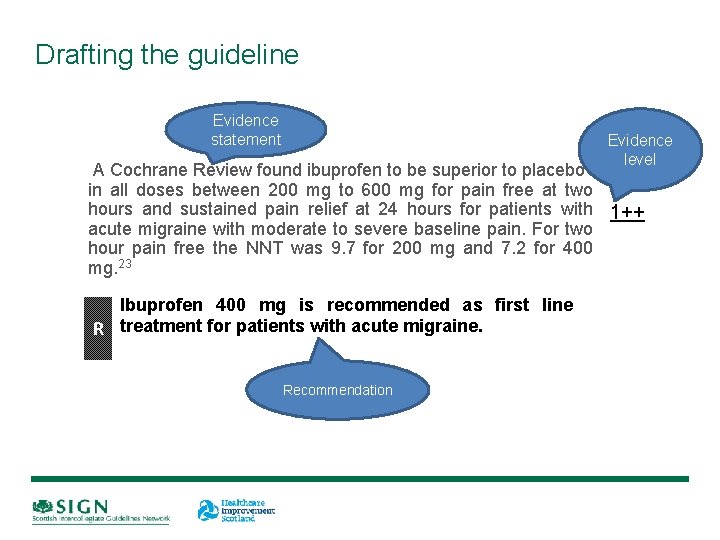
Drafting the guideline Evidence statement Evidence level A Cochrane Review found ibuprofen to be superior to placebo in all doses between 200 mg to 600 mg for pain free at two hours and sustained pain relief at 24 hours for patients with 1++ acute migraine with moderate to severe baseline pain. For two hour pain free the NNT was 9. 7 for 200 mg and 7. 2 for 400 mg. 23 Ibuprofen 400 mg is recommended as first line R treatment for patients with acute migraine. Recommendation

Additional content • Recommendations for further research • Provision of information for patients and carers • Audit points • Cost impact analysis (if needed)

Consultation www. sign. ac. uk • open consultation • peer review • early 2018

Publication and implementation August 2018 www. sign. ac. uk

• www. sign. ac. uk • ailsa. stein@nhs. net
 Ailsa raeburn
Ailsa raeburn Ailsa crum
Ailsa crum All the signs for driving
All the signs for driving Nursing diagnosis for community health
Nursing diagnosis for community health Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Second phase of nursing process
Second phase of nursing process Objectives of nursing process
Objectives of nursing process Difference between dementia and delirium
Difference between dementia and delirium Francisco fernandez md
Francisco fernandez md Difference between delirium and dementia table
Difference between delirium and dementia table Waikato stormwater management guideline
Waikato stormwater management guideline Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Lucie big delirium
Lucie big delirium Dr rose dinda martini
Dr rose dinda martini Nash delirium
Nash delirium Terminalt delirium
Terminalt delirium Escala rass
Escala rass Delirium care pathways
Delirium care pathways Delirium definition
Delirium definition Delirium definition
Delirium definition Energy transfer theory
Energy transfer theory