Shock Processing of Ice Mixtures in Protoplanetary Disks













- Slides: 29

Shock Processing of Ice Mixtures in Protoplanetary Disks George Hassel & Wayne Roberge ghassel@mps. ohio-state. edu RPI Physics Department New York Center for Studies on the Origins of Life http: //www. origins. rpi. edu Astro. Surf 2007 Meeting, June 15, 2007 1

Shock Processing of Ices in Protoplanetary Disks n Purpose of Study: Detailed analysis of ice processing by shocks u Implications for the early Earth u n Research Team: Wayne Roberge & Glenn Ciolek u Doug Whittet & Sachin Shenoy u Origins of Life NSCORT Group u 2

Outline of Project Shocks widespread in protoplanetary disks (Desch & Connolly 2002, Boss 2002, Cuzzi & Alexander 2005) v Icy mantles processed in disk shocks v Formation of comets from resulting ice v Delivery to early Earth ? ocean water and biogenic molecules 3

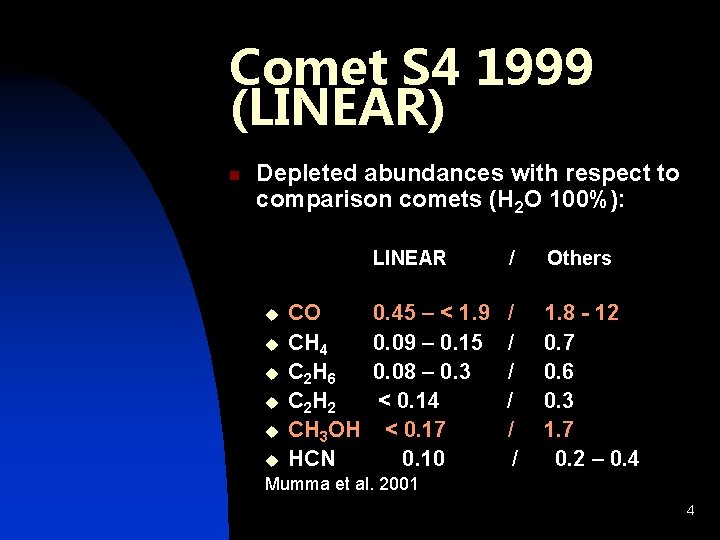
Comet S 4 1999 (LINEAR) n Depleted abundances with respect to comparison comets (H 2 O 100%): u u u CO CH 4 C 2 H 6 C 2 H 2 CH 3 OH HCN LINEAR / Others 0. 45 – < 1. 9 0. 09 – 0. 15 0. 08 – 0. 3 < 0. 14 < 0. 17 0. 10 / / / 1. 8 - 12 0. 7 0. 6 0. 3 1. 7 0. 2 – 0. 4 Mumma et al. 2001 4

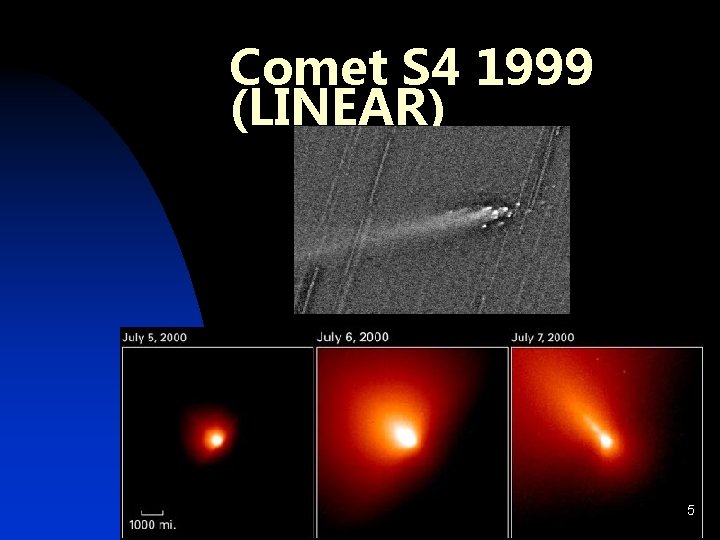
Comet S 4 1999 (LINEAR) 5

Astro. Surf Relevance “Chemistry” models dep. heavily on surface processes: n Binding energy n Desorption kinetics n Crystallization rates 6

A Shock is: • A disturbance moving faster than the signal speed in a medium 7

A Shock is: • Turns organized motion into random motion behind the shock front 8

A Shock is: • Frictional Drag Heating between gas and dust 9

Shock Processing Overview n Disk Model – Hydro Inputs n Hydro / RT Simulation u u u n Temperature Density Velocity Profiles Ice Mantle Processing: u Removal u Crystallization u Guest Retention / Exclusion 10

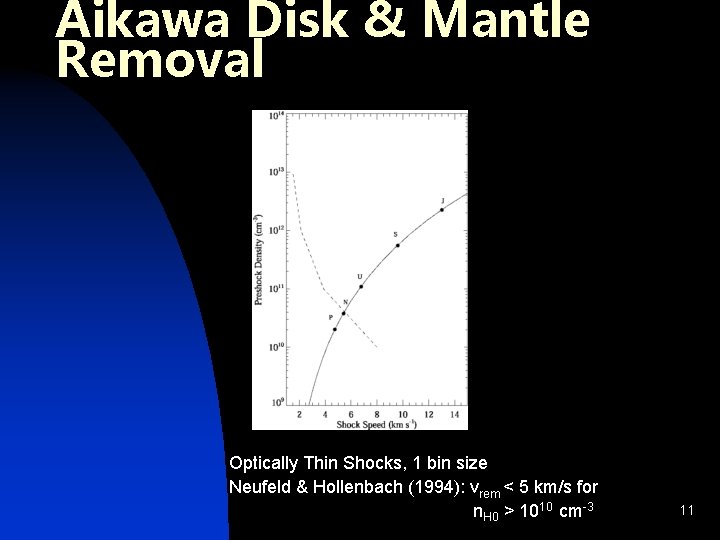
Aikawa Disk & Mantle Removal Optically Thin Shocks, 1 bin size Neufeld & Hollenbach (1994): vrem < 5 km/s for n. H 0 > 1010 cm-3 11

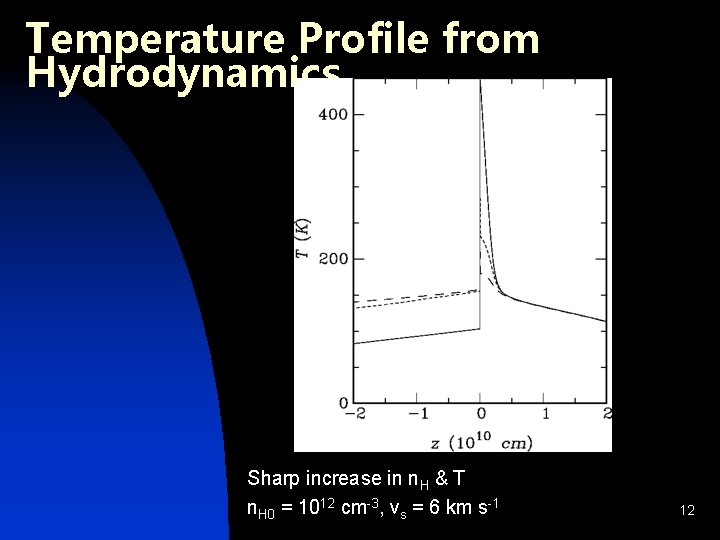
Temperature Profile from Hydrodynamics Sharp increase in n. H & T n. H 0 = 1012 cm-3, vs = 6 km s-1 12

Mantle Processing n Ice Removal / Re-accretion n Pure Water Model: u u n Crystallization of Water Dominant component Water + 1 Guest: u u u Exclusion? Retention? Clathrate Hydrate Formation? 13

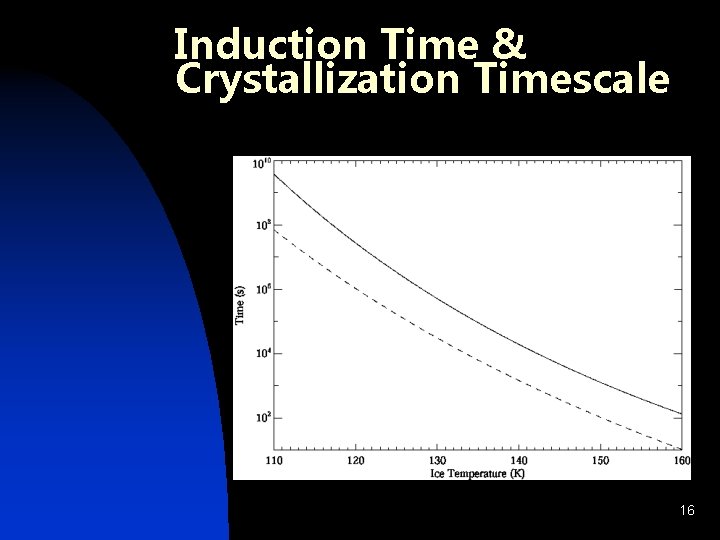
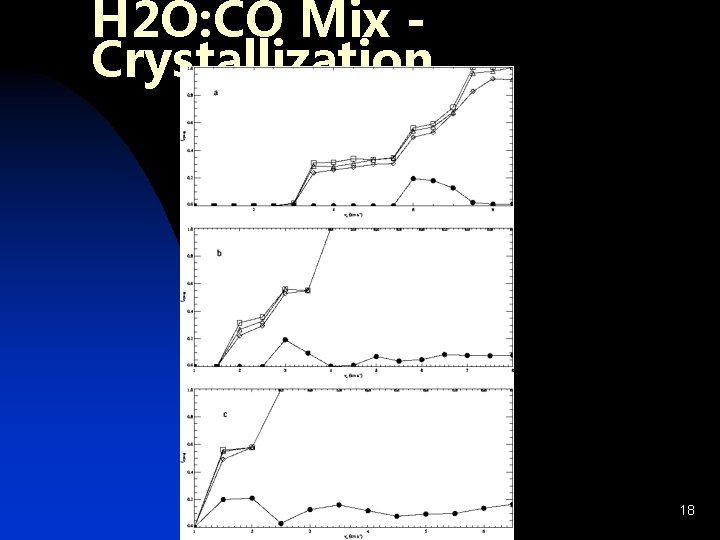
Water Crystallization n Water is dominant component of ice n Phase Change: u Amorphous Ice – retains guests u Cubic Crystalline – excludes guests u Clathrate Hydrate – retains select guests 14

Water Crystallization: Crystallizaton and Growth n Removal: Mobility of Reaccreting Surface Molecules u Kouchi et al. 1994 u n Otherwise: Classical Nucleation Theory u Jenniskens & Blake 1996 u 15

Induction Time & Crystallization Timescale 16

Results! 17

H 2 O: CO Mix Crystallization 18

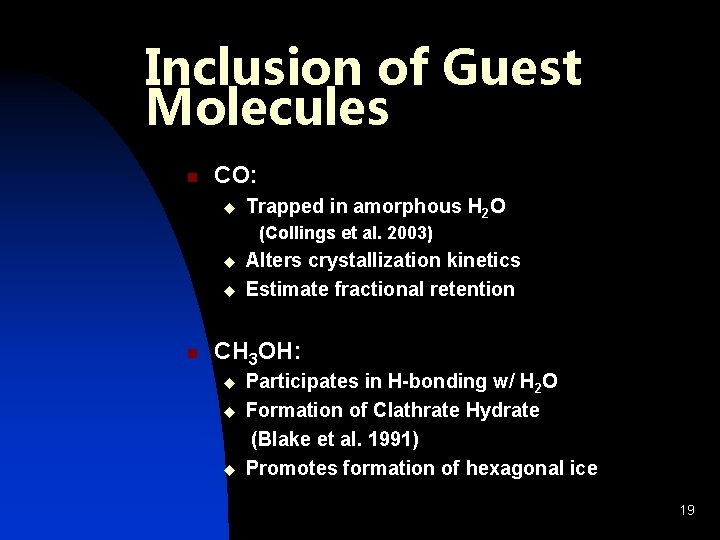
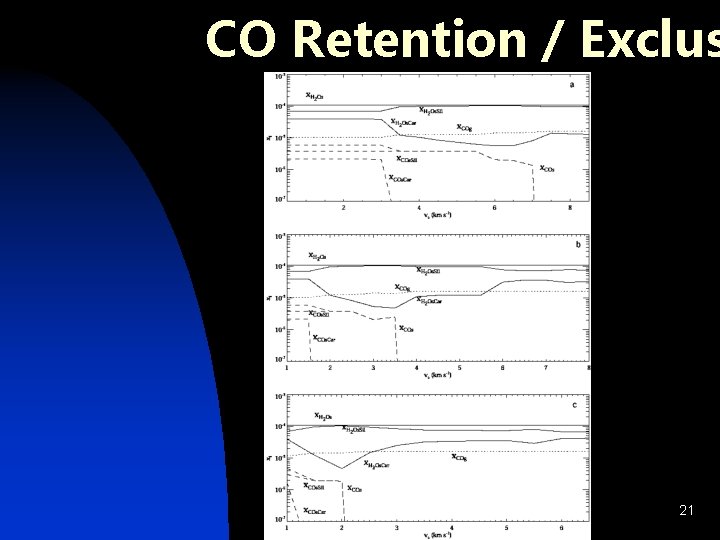
Inclusion of Guest Molecules n CO: u Trapped in amorphous H 2 O (Collings et al. 2003) u u n Alters crystallization kinetics Estimate fractional retention CH 3 OH: u u u Participates in H-bonding w/ H 2 O Formation of Clathrate Hydrate (Blake et al. 1991) Promotes formation of hexagonal ice 19

H 2 O: CH 3 OH Mix – Clathrate Fraction 20

CO Retention / Exclus 21

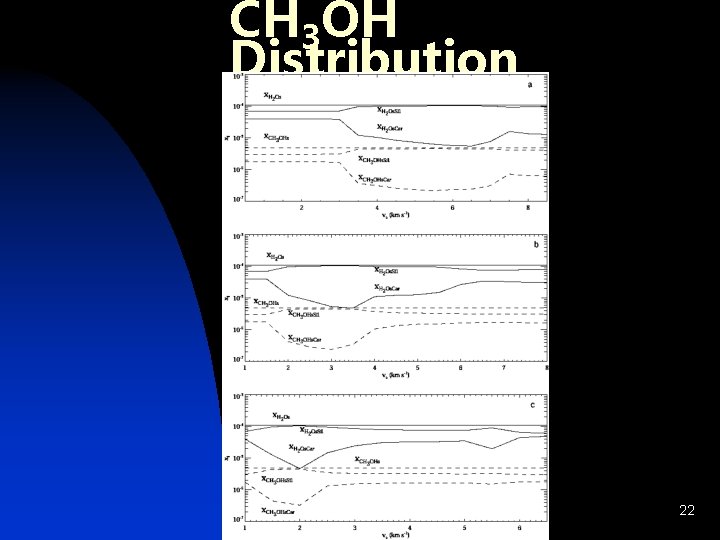
CH 3 OH Distribution 22

Summary of Results Crystallization efficient if n vs > vremoval n u small range of speeds for vs < vremoval where crystallization possible from nucleation and growth u vremoval decreases with increasing n. H 0 – “easier” to remove & crystallize mantle at higher densities u shocks due to gravitational instabilities: vs may be < VKepler due to oblique incidence (Pickett et al 2003) CO: u u n removed with H 2 O retained (partially) for no/partial mantle removal CH 3 OH: u formation of clathrate hydrate predicted where crystallization is predicted in pure ice – may affect 23 detectability

Future Projects n n Extension of Shock Processing with Multiple Grain Size / Composition Shock-Enhanced Chemistry: u D/H exchange Shock effects on refractory material: u crystallization of silicates Observational Properties 24

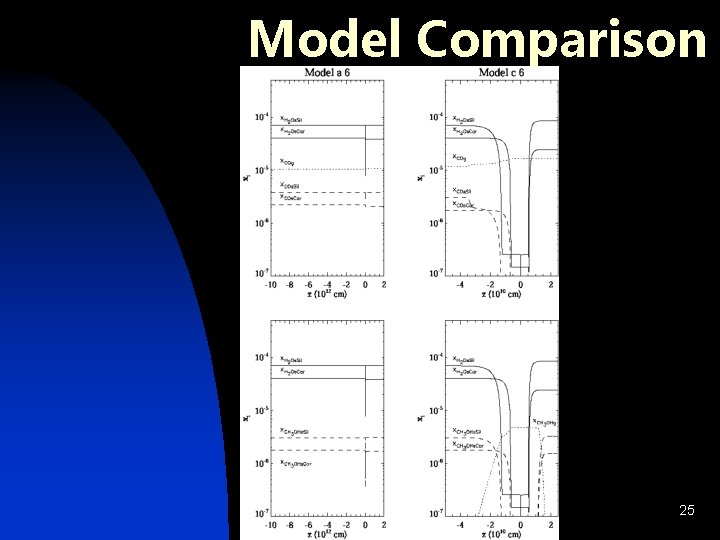
Model Comparison 25

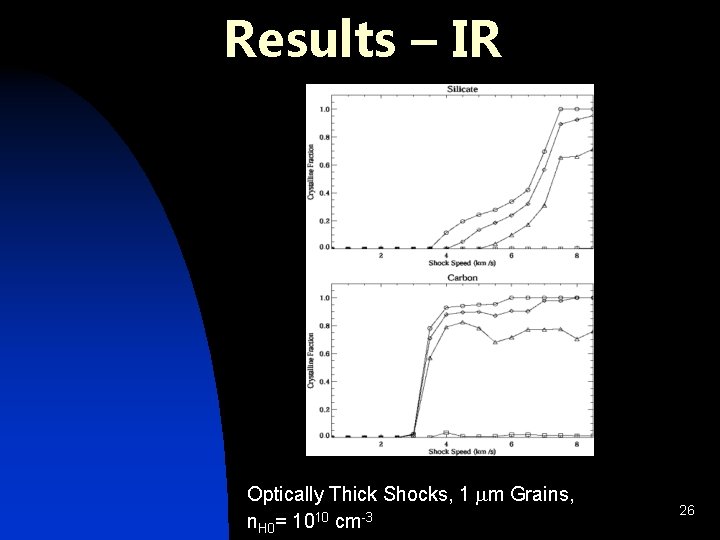
Results – IR Optically Thick Shocks, 1 mm Grains, n. H 0= 1010 cm-3 26

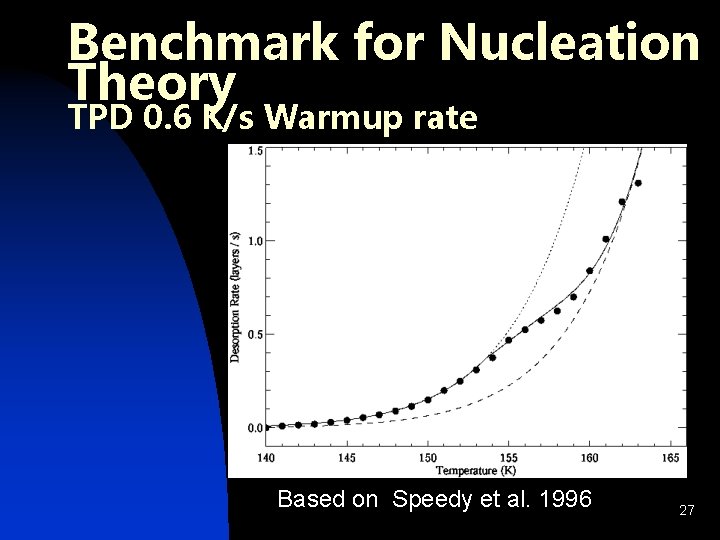
Benchmark for Nucleation Theory TPD 0. 6 K/s Warmup rate Based on Speedy et al. 1996 27

Benchmark for Nucleation Theory T = 146 K Annealing Based on Safarik et al. 2003 28

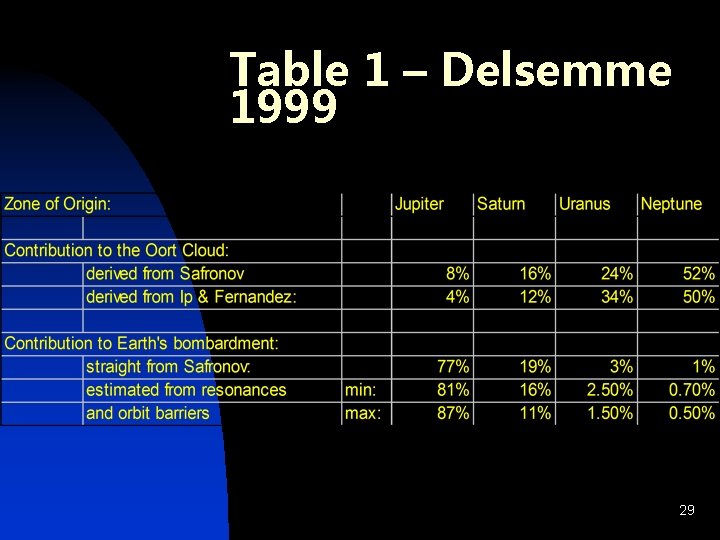
Table 1 – Delsemme 1999 29
 Protoplanetary disk
Protoplanetary disk Spinal shock symptoms
Spinal shock symptoms Spinal shock vs neurogenic shock
Spinal shock vs neurogenic shock Subacute combined degeneration of the cord
Subacute combined degeneration of the cord Shock normovolemico
Shock normovolemico Spinal shock vs neurogenic shock
Spinal shock vs neurogenic shock Clear ice vs rime ice
Clear ice vs rime ice Clear ice vs rime ice
Clear ice vs rime ice Disks of polycarbonate plastic from a supplier are analyzed
Disks of polycarbonate plastic from a supplier are analyzed Redundant arrays of independent disks
Redundant arrays of independent disks Holger brock
Holger brock Floppy disk
Floppy disk Azure vm ssd
Azure vm ssd Tower of hanoi 4 disks
Tower of hanoi 4 disks Conquest data disks
Conquest data disks Daisy wheel printer
Daisy wheel printer Disks and tapes can be stored ------- a library. eng101
Disks and tapes can be stored ------- a library. eng101 Redundant array of inexpensive disk
Redundant array of inexpensive disk Types of external memory
Types of external memory Raid 5 nedir
Raid 5 nedir Computer parts labeling
Computer parts labeling Define point processing
Define point processing Batch processing and interactive processing
Batch processing and interactive processing Bottom-up processing example
Bottom-up processing example Histogram processing in digital image processing
Histogram processing in digital image processing Point processing
Point processing Point processing and neighbourhood processing
Point processing and neighbourhood processing Digital image processing
Digital image processing Parallel processing vs concurrent processing
Parallel processing vs concurrent processing Top down processing
Top down processing