Shielding Effect The shielding effect is the reduction




- Slides: 18

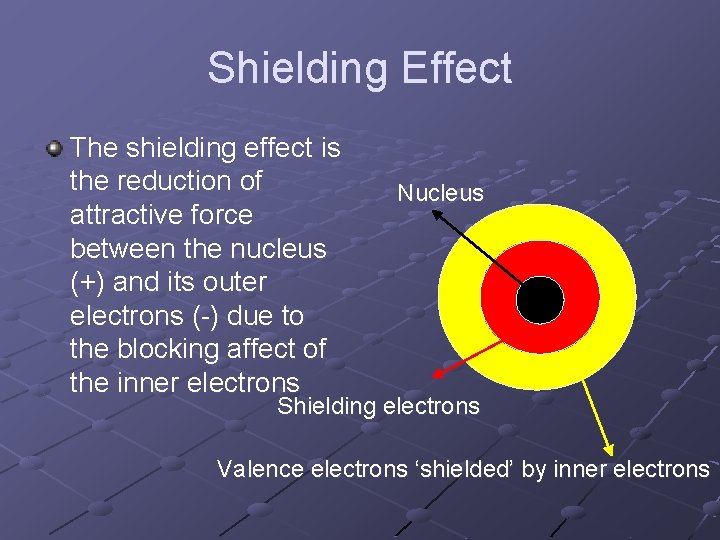
Shielding Effect The shielding effect is the reduction of attractive force between the nucleus (+) and its outer electrons (-) due to the blocking affect of the inner electrons Nucleus Shielding electrons Valence electrons ‘shielded’ by inner electrons

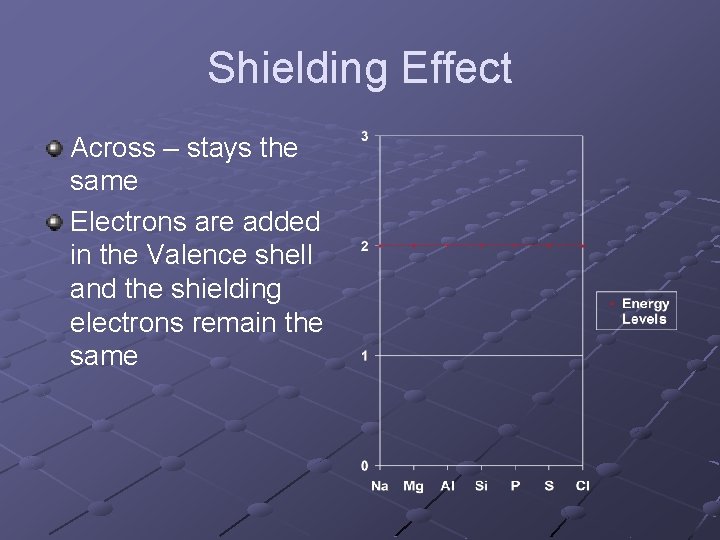
Shielding Effect Across – stays the same Electrons are added in the Valence shell and the shielding electrons remain the same

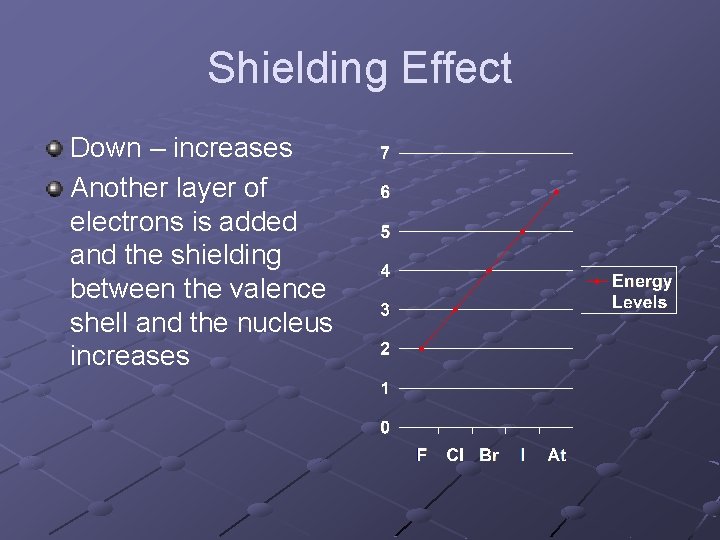
Shielding Effect Down – increases Another layer of electrons is added and the shielding between the valence shell and the nucleus increases

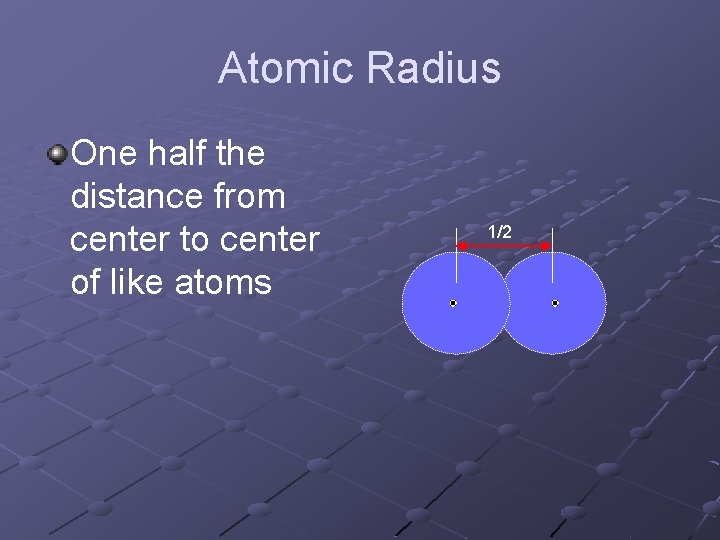
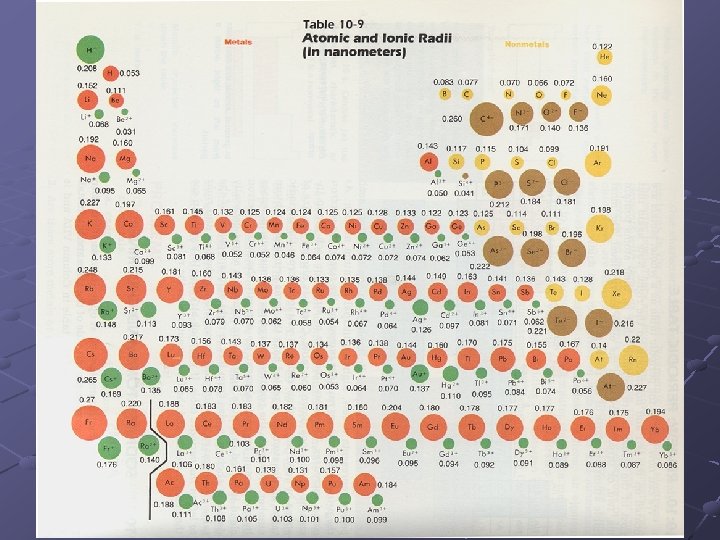
Atomic Radius One half the distance from center to center of like atoms 1/2

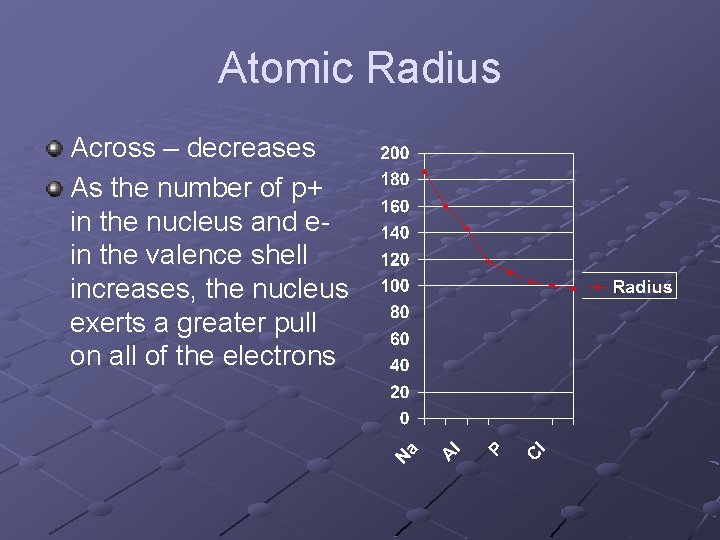
Atomic Radius Across – decreases As the number of p+ in the nucleus and ein the valence shell increases, the nucleus exerts a greater pull on all of the electrons

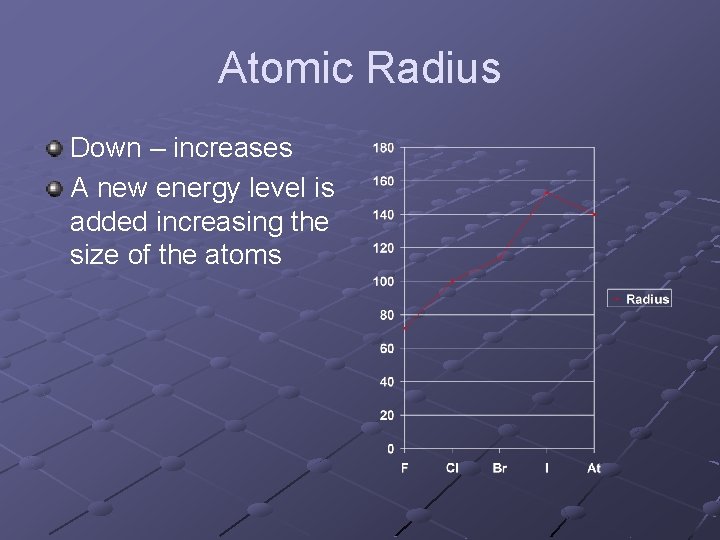
Atomic Radius Down – increases A new energy level is added increasing the size of the atoms

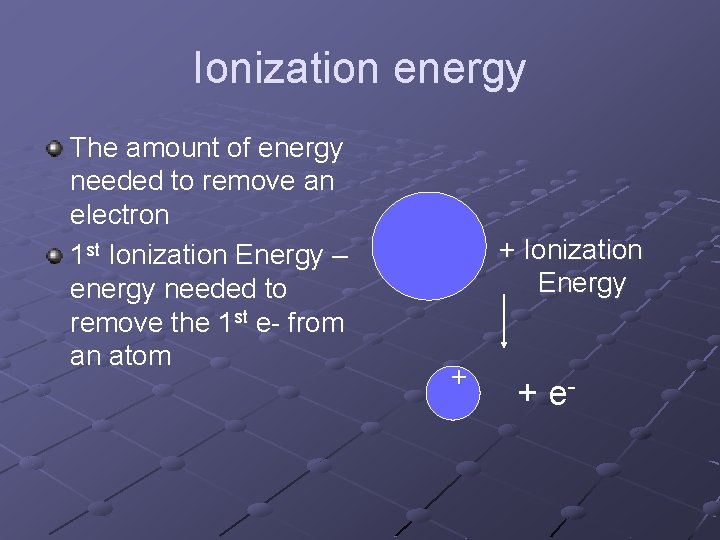
Ionization energy The amount of energy needed to remove an electron 1 st Ionization Energy – energy needed to remove the 1 st e- from an atom + Ionization Energy + + e-

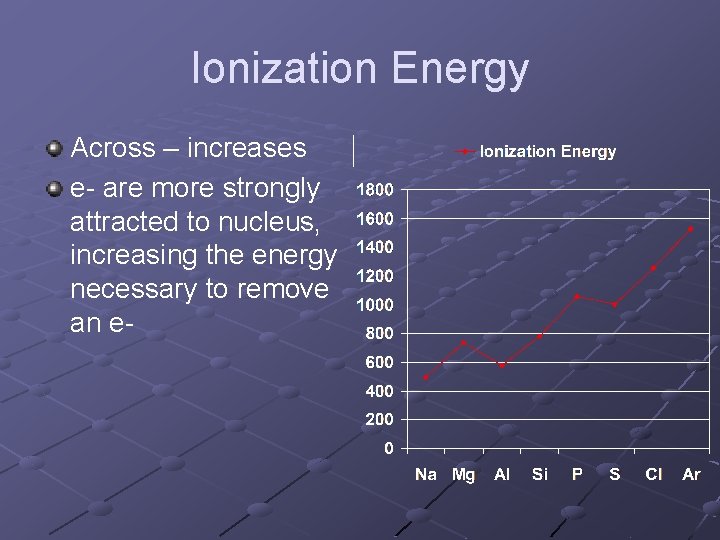
Ionization Energy Across – increases e- are more strongly attracted to nucleus, increasing the energy necessary to remove an e-

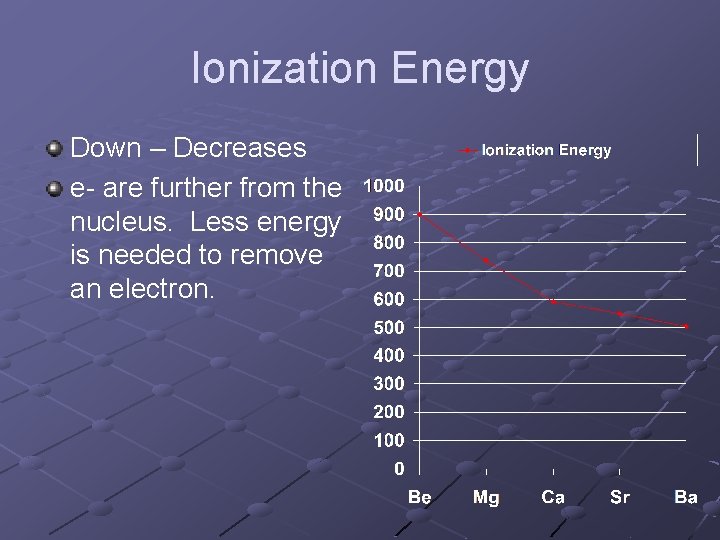
Ionization Energy Down – Decreases e- are further from the nucleus. Less energy is needed to remove an electron.

Ionic Radius of an ion -- the size of an ion is different than the size of the atom it came from. If an atom loses an e- then it will have a pos. charge and is called a CATION. If an atom gains an e- then it will have a neg. charge and is called an ANION.

Ionic Radius Cations will be smaller than the original atom. This is due to a stronger nuclear pull. Anions will be larger than the original atom. The additional e- goes into the same energy level and the e- will repel each other and spread out.


Electronegativity The tendency for an atom to attract electrons in a bond.

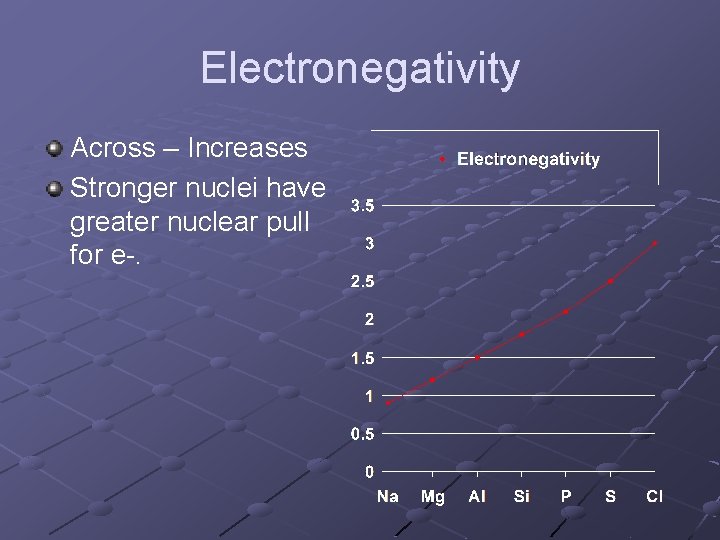
Electronegativity Across – Increases Stronger nuclei have greater nuclear pull for e-.

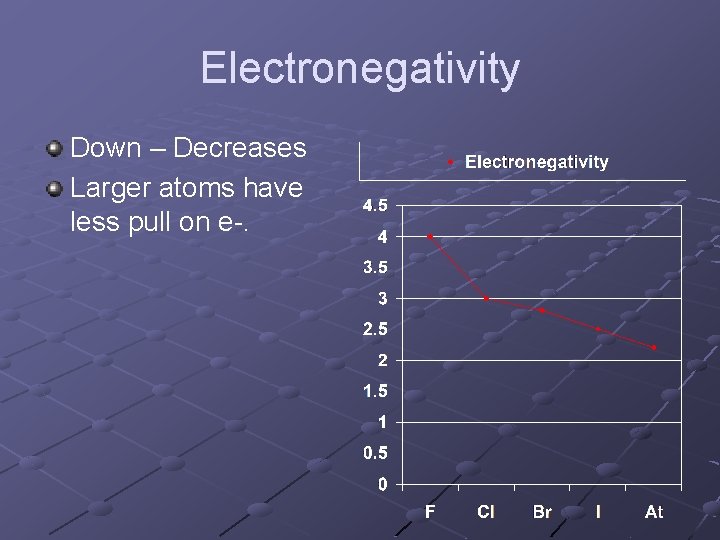
Electronegativity Down – Decreases Larger atoms have less pull on e-.

Electron Affinity The energy change when an electron is added to a neutral atom to form a negative ion Higher e- affinity = easier to add an e- = stronger nucleus

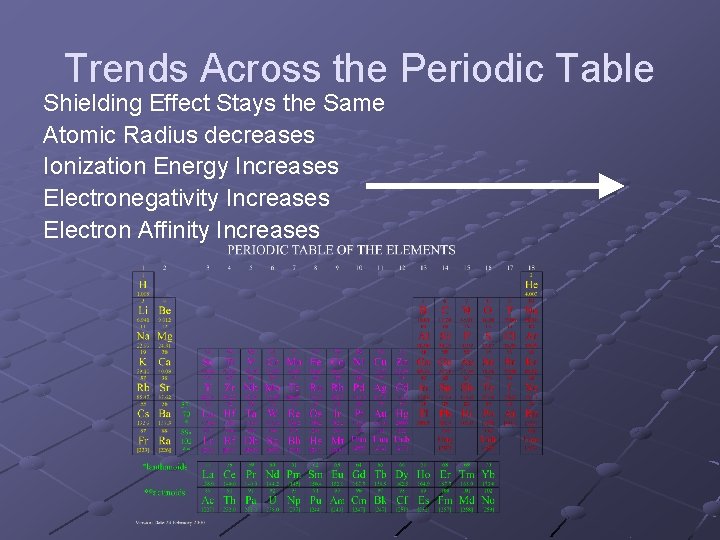
Trends Across the Periodic Table Shielding Effect Stays the Same Atomic Radius decreases Ionization Energy Increases Electronegativity Increases Electron Affinity Increases

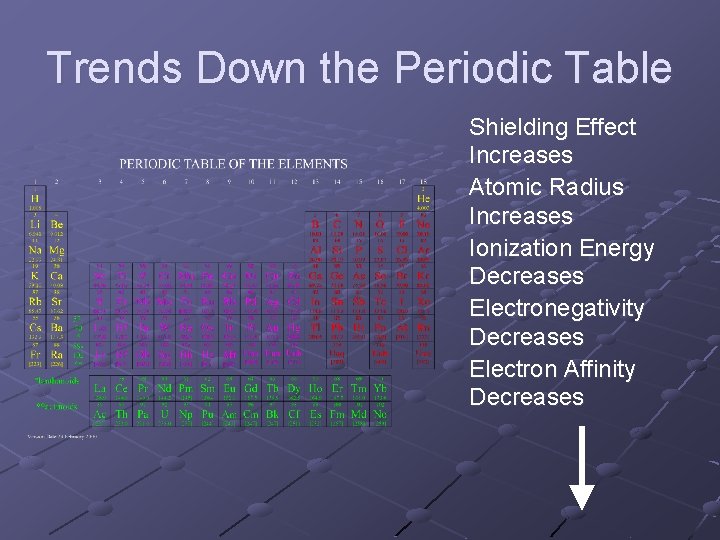
Trends Down the Periodic Table Shielding Effect Increases Atomic Radius Increases Ionization Energy Decreases Electronegativity Decreases Electron Affinity Decreases