Shapes of Molecules Intermolecular Forces Shapes of covalent







- Slides: 22

Shapes of Molecules & Intermolecular Forces

Shapes of covalent molecules • VSEPR – Valence Shell Electron Pair Repulsion • Use this to predict shapes of covalent molecules • Count number of bond pairs and lone pairs • Predict shape

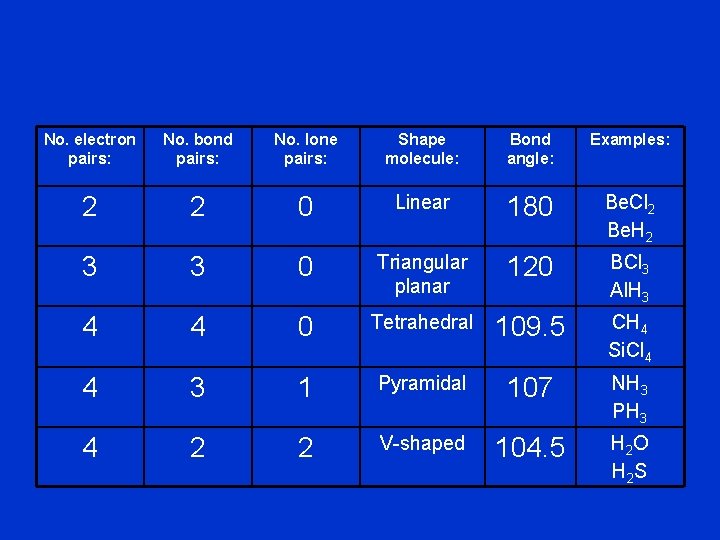
No. electron pairs: No. bond pairs: No. lone pairs: Shape molecule: Bond angle: Examples: 2 2 0 Linear 180 Be. Cl 2 Be. H 2 3 3 0 Triangular planar 120 BCl 3 Al. H 3 4 4 0 Tetrahedral 109. 5 CH 4 Si. Cl 4 4 3 1 Pyramidal 107 NH 3 PH 3 4 2 2 V-shaped 104. 5 H 2 O H 2 S

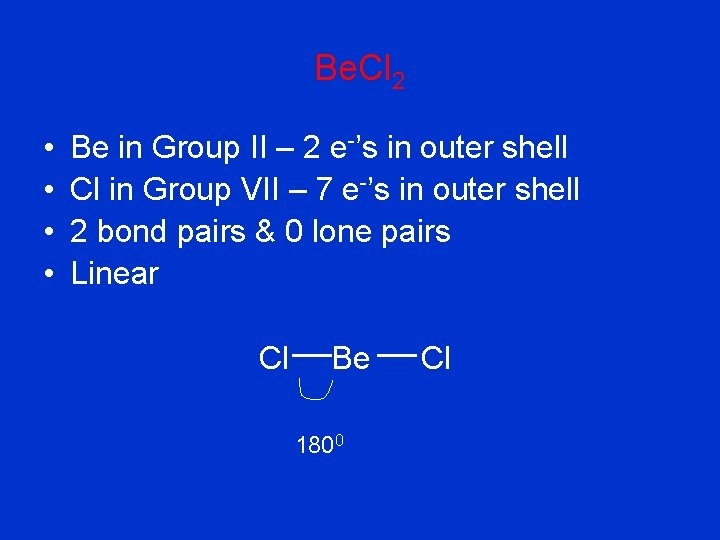
Be. Cl 2 • • Be in Group II – 2 e-’s in outer shell Cl in Group VII – 7 e-’s in outer shell 2 bond pairs & 0 lone pairs Linear Cl Be 1800 Cl

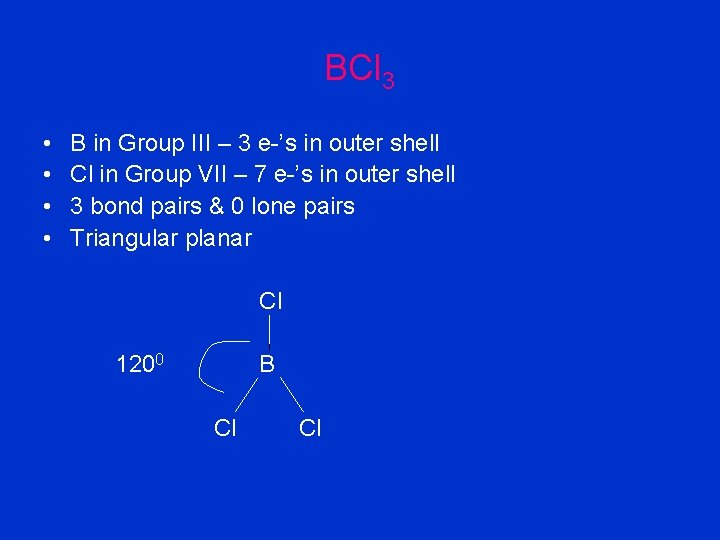
BCl 3 • • B in Group III – 3 e-’s in outer shell Cl in Group VII – 7 e-’s in outer shell 3 bond pairs & 0 lone pairs Triangular planar Cl 1200 B Cl Cl

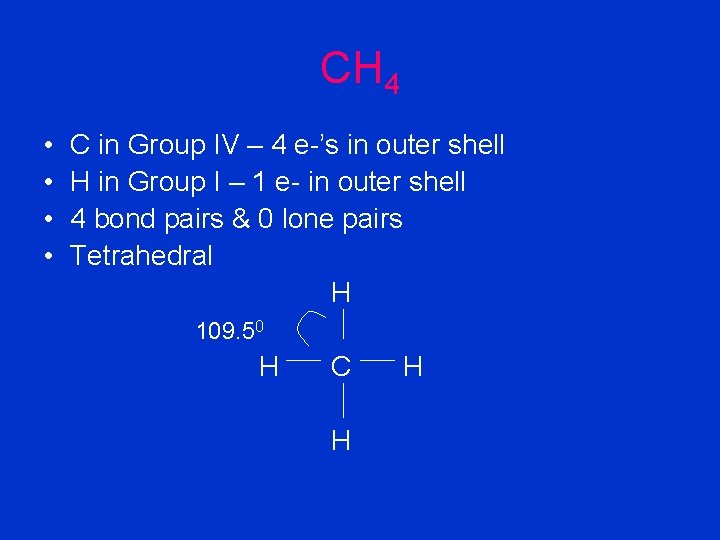
CH 4 • • C in Group IV – 4 e-’s in outer shell H in Group I – 1 e- in outer shell 4 bond pairs & 0 lone pairs Tetrahedral H 109. 50 H C H H

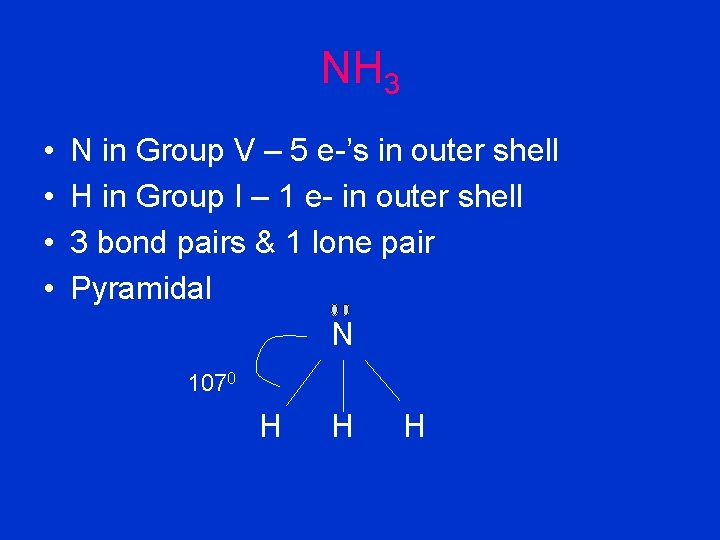
NH 3 • • N in Group V – 5 e-’s in outer shell H in Group I – 1 e- in outer shell 3 bond pairs & 1 lone pair Pyramidal N 1070 H H H

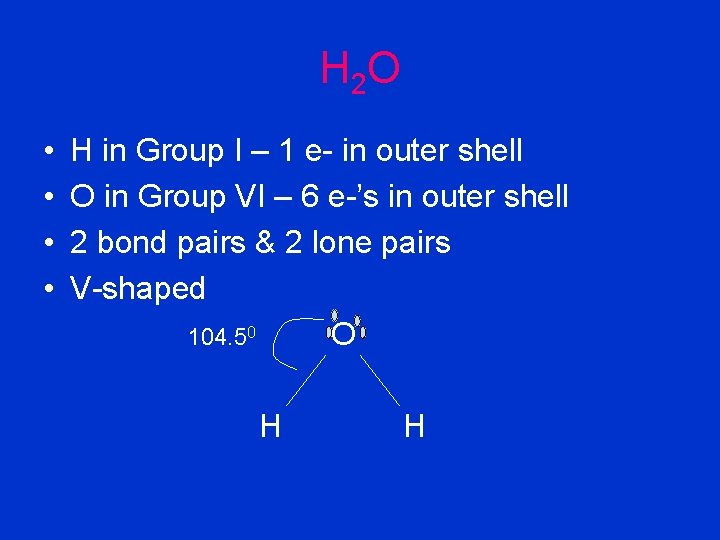
H 2 O • • H in Group I – 1 e- in outer shell O in Group VI – 6 e-’s in outer shell 2 bond pairs & 2 lone pairs V-shaped 104. 50 O H H

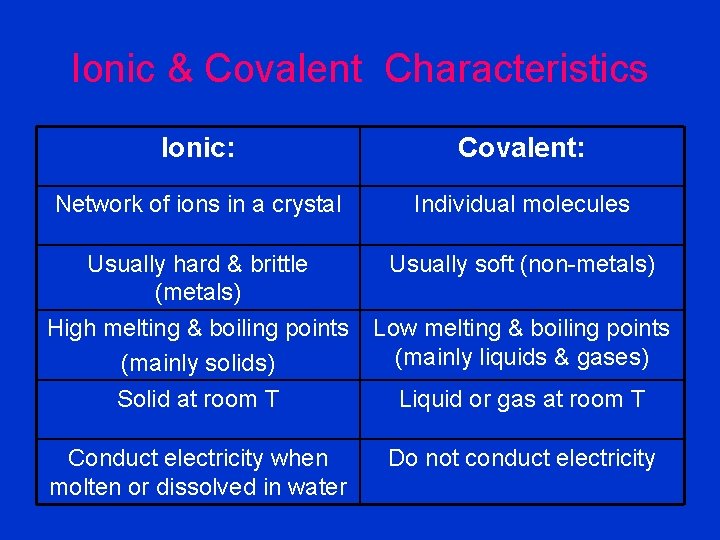
Ionic & Covalent Characteristics Ionic: Covalent: Network of ions in a crystal Individual molecules Usually hard & brittle (metals) Usually soft (non-metals) High melting & boiling points (mainly solids) Solid at room T Low melting & boiling points (mainly liquids & gases) Conduct electricity when molten or dissolved in water Do not conduct electricity Liquid or gas at room T

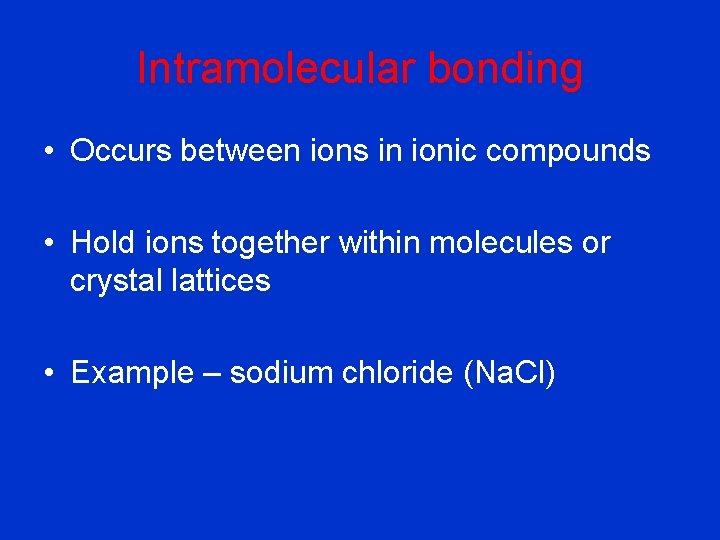
Intramolecular bonding • Occurs between ions in ionic compounds • Hold ions together within molecules or crystal lattices • Example – sodium chloride (Na. Cl)

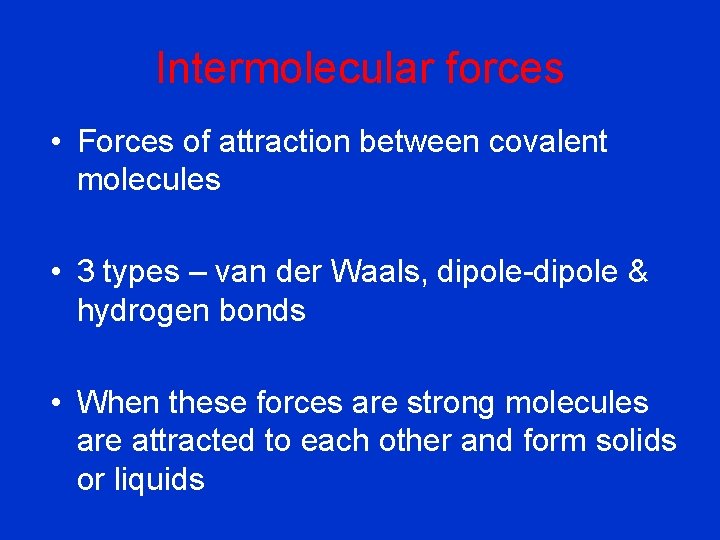
Intermolecular forces • Forces of attraction between covalent molecules • 3 types – van der Waals, dipole-dipole & hydrogen bonds • When these forces are strong molecules are attracted to each other and form solids or liquids

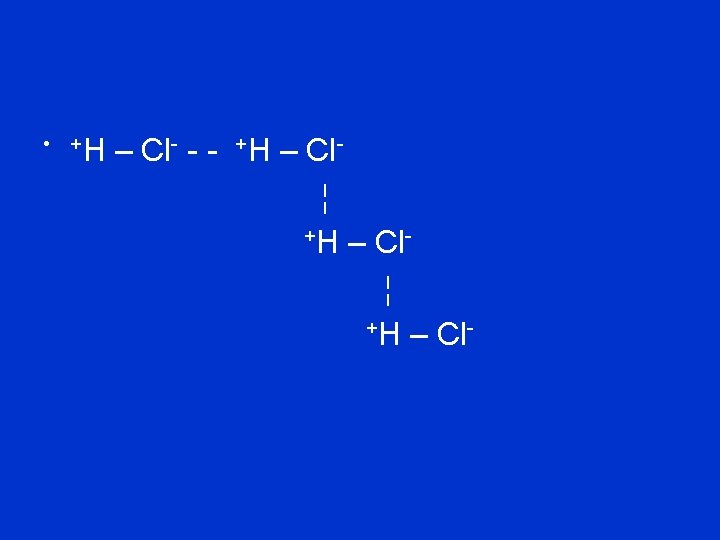
Van der Waals forces • Weakest intermolecular force • Exist between neutral atoms • As electrons move within atoms temporary dipoles can occur

• Opposite ends of these temporary dipoles attract each other • Temporary so not very strong • +H – H - - - +H – H ¦ +H – H -

Dipole-dipole forces • Stronger than van der Waals • In polar molecules the positive pole of one molecule is attracted to the negative pole of another • Permanent dipoles so quite strong


Hydrogen bonding • Strongest intermolecular force • When a H atom is bonded to small strongly electronegative atoms like N, O or F it gains a partial positive charge • This positive H atom is then attracted to the negative poles in other molecules forming a hydrogen bond

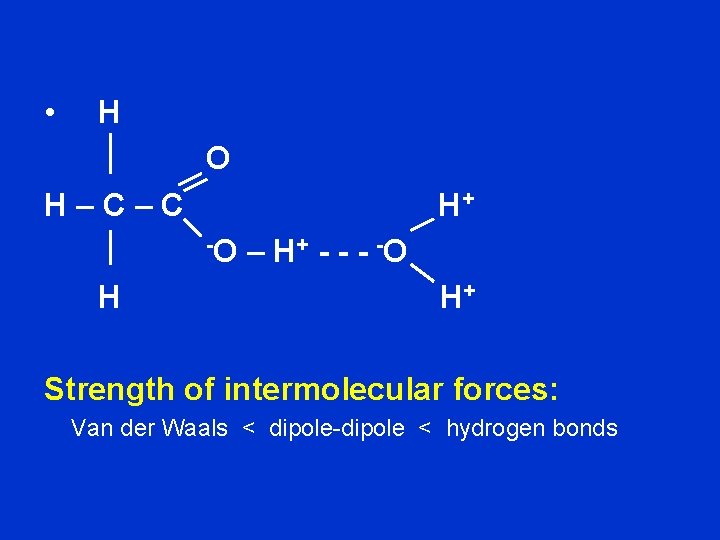
• H O H–C–C H+ -O H – H + - -O H+ Strength of intermolecular forces: Van der Waals < dipole-dipole < hydrogen bonds

Boiling Points • Boiling points are increased when there are intermolecular forces present • Examples – (a) H 2 and O 2: Both molecules are non-polar so can only have van der Waals forces O 2 has higher b. p. because its heavier +H – H+ Mr = 2 -O == OMr = 16

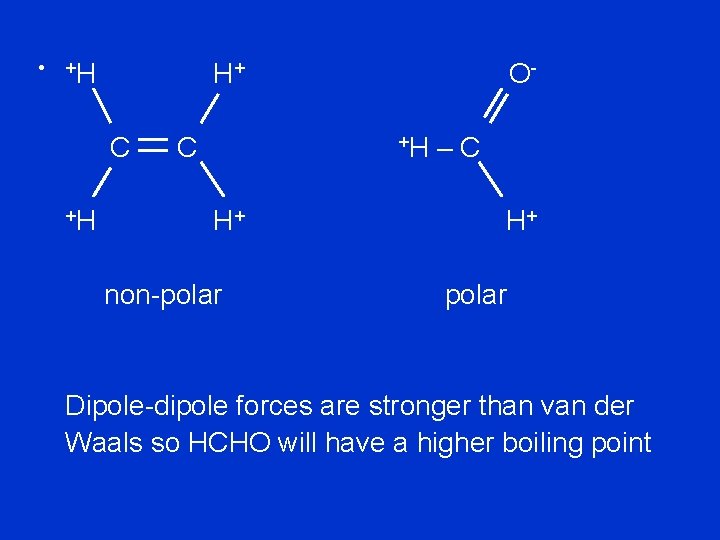
(b) C 2 H 4 and HCHO: C 2 H 4 is non-polar so can only have van der Waals forces HCHO has a C == O bond so is polar so can have dipole-dipole forces

• +H H+ C +H C O+H H+ non-polar –C H+ polar Dipole-dipole forces are stronger than van der Waals so HCHO will have a higher boiling point

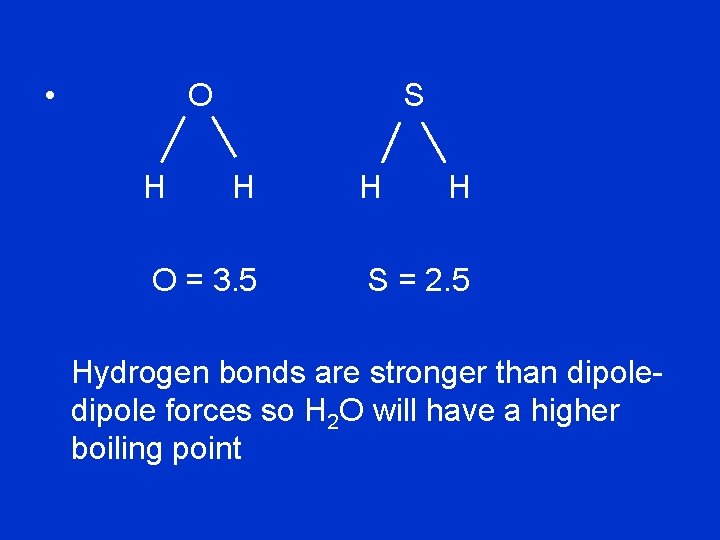
(c) H 2 O and H 2 S: H 2 O has a polar O --- H bond and O is strongly electronegative so can form hydrogen bonds H 2 S has a polar S --- H bond but S is not very electronegative so can only form dipole-dipole forces and not hydrogen bonds

• O H S H O = 3. 5 H H S = 2. 5 Hydrogen bonds are stronger than dipole forces so H 2 O will have a higher boiling point