Shantha Biotechnics Limited 1 Mission Statement To develop

Shantha Biotechnics Limited 1

Mission Statement To develop, produce and market cost-effective human healthcare products that conform to international standards of high order. 2

Story of Shantha • When Shantha was founded in 1993, Biopharmaceutical sector was unknown in the country. No funding – No skill set – No experience • First Company to indigenously develop and manufacture r-DNA based Hepatitis-B vaccine (Shanvac-B) in 1997 • First Company to place India on the map of Genetic Engineering World • Was awarded the first National Technology Award in 1999 for “Successful development and commercialization of indigenous technology” 3
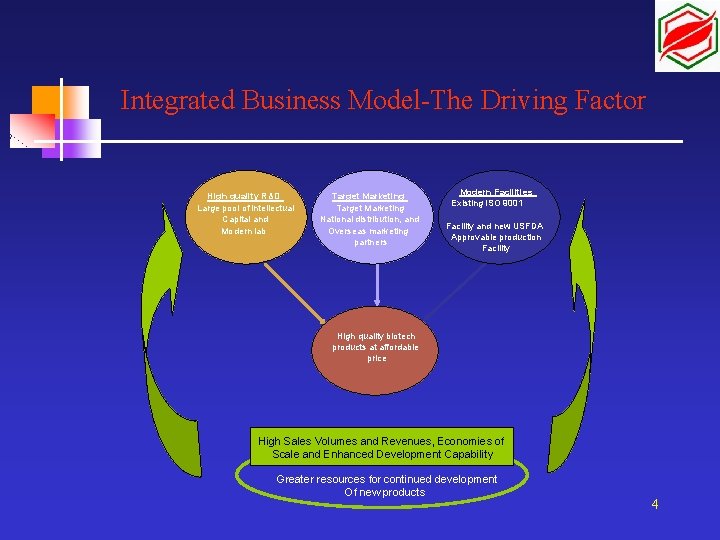
Integrated Business Model-The Driving Factor High quality R&D Large pool of intellectual Capital and Modern lab Target Marketing National distribution, and Overseas marketing partners Modern Facilities Existing ISO 9001 Facility and new USFDA Approvable production Facility High quality biotech products at affordable price High Sales Volumes and Revenues, Economies of Scale and Enhanced Development Capability Greater resources for continued development Of new products 4

Business Model …. . contd Business Segments • Vaccines – Recombinant, Conventional, Combos, Conjugates, Viral, Newer Delivery Systems • Therapeutic Proteins – modified Human Proteins Hormones, Cytokines, • Antibodies – Fully Human, Novel • Contract Services Shantha is a focussed Biopharmaceutical 5

Core Values • Create a congenial work environment • Strict conformity to current Good Manufacturing Practices (c. GMP) and current Good Laboratory Practices (c. GLP). • Continued R&D efforts for continuous improvement and new solutions. • Create a scientific base to promote innovative R&D programmes • To seek active collaborations / partnerships from leading organisations in India and abroad 6 to complement our strengths.
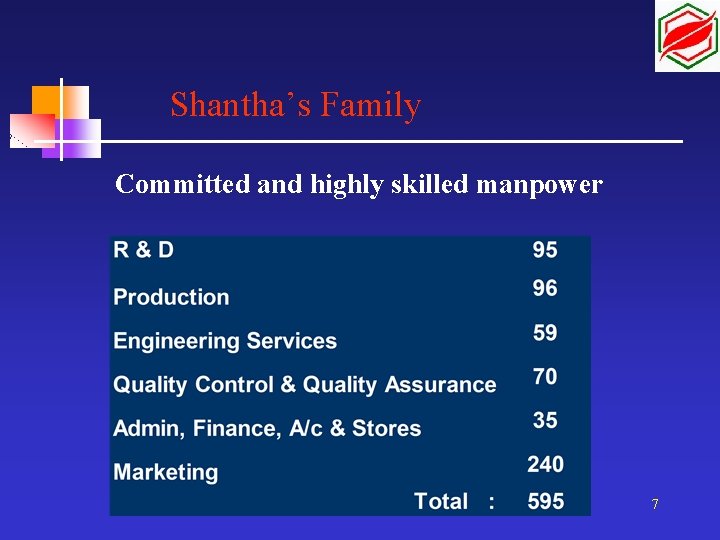
Shantha’s Family Committed and highly skilled manpower 7
R&D - Place of Pride Core Competence • Molecular Biology • Microbial Fermentation • Down stream process development for biologicals • Protein Purification • Formulation & Lypholization • Mammalian Cell Culture • Development of Monoclonal Antibodies 8

Facilities • Aesthetically designed modern facility with a total built-up area of 170, 000 sq. ft. • 66, 000 sq. ft. of Production Area • Provided with state-of-the-art computer controlled equipments • WHO (Geneva) pre-qualified Hepatitis B vaccine production facility 9

Facilities …contd. • Engineering Services consisting of 14, 650 sq. ft. of built-up area with modern support facilities • Quality Control laboratory extending over 8, 000 sq. ft. area with modern analytical equipments • 40, 000 sq. ft. of Research and Development Laboratory • Animal House conforming to Good Laboratory Practices with a built-up area of 17, 000 sq. ft. • 8, 000 sq. ft. of Stores area conforming to the c. GMP requirements 10

New facility Under Construction Four c. GMP suites in consultation with LSMW, German Microbial Fermentation & Purification Suite Microbial Fill & Finish Viral Vaccine Cell Culture Viral Vaccine Fill & Finish 11

Products Launched 12

Shanvac-B - 1997 Shanvac - B • Shantha’s first product and India’s first indigenous r. DNA Hepatitis-B vaccine • Produced from genetically engineered yeast Pichia pastoris, a new generation host. • Shown to have superior immunogenicity in clinical trials. • India’s first Hepatitis-B vaccine to get WHOGeneva pre-qualification. • Over 100 Million doses administered since launch (August, 1997) with a good efficacy and safety 13

Shanferon - 2002 • Recombinant Human Interferon Alpha 2 b launched in 2002 • India’s first and World’s only interferon that is derived from Pichia pastoris for human use • PCT Process patent in National Phase • 14 More than 3000 Patients benefited from Shanferon

Shankinase - 2003 • State of the Art Recombinant Streptokinase conforming to International Standards • Efficacy as per International references established in Comparative Multicentric Clinical Trial 15

Shanpoetin - 2005 • Recombinant Human Erythropoietin • Expressed in CHO cells • Conforming to European Pharmacopeial standards • Produced in Serum free media in fermentor • Established safety and Multicentric clinical study efficacy in 16

DTP-Hep. B Combo Vaccine 2005 • Indigenously developed Combo vaccine with Hep. B • Highly Safe and Immunogenic as established in Multicentric Phase III trials • Seroconversion of Hepatitis-B vaccine in combination same as in Monovalent form 17

Vaccines to be Launched in next 12 months • DPT • TT • HIB and its Combination with DPTH 18

Vaccine – In-house Research Projects • HPV • DTa. P • DPT free from animal source material • Single Shot Hepatitis B 19

Vaccine – Collaborative Projects • Cholera with IVI, Korea • Rotavirus with NIH • Heat Stable Vaccine, Cambridge, UK 20

Research Collaborations – India • • Centre for Cellular & Molecular Biology Indian Institute of Chemical Technology National Institute of Immunology International Centre for Genetic Engineering & Biotechnology All Indian Institute of Medical Sciences Indian Institute of Science Bhabha Atomic Research Centre National Centre for Cell Sciences 21

Research Collaborations - Global • International Vaccine Institute, South Korea • National Institute of Health, USA • John Hopkins University, USA • Meristem Therapeutics, France • University of London, U. K. • National Cancer Institute, USA • Aeras Foundation, USA 22

Major Awards among Many… • “DSIR Award” for “Best R&D Efforts” for the years 1998 & 2003 • “National Technology Award” for “Successful development and commercialisation of technology” for the years 1999 & 2003 • Ernst & Young’s-“Entrepreneur of the year Award” for the healthcare industry for the year 1999 23

National Technology Award 24

Padma Bhushan Award – 2005 from Hon’ble President of India 25

26

Thank you for your time. Please visit us at www. Shanthabiotech. com Mailing Address Shantha Biotechnics Ltd. 3 rd Floor, Serene Chambers, Road No. 7, Banjara Hills Hyderabad - 500 034 A. P, India Ph. : +91 -40 -23548507/3010, Fax: +91 -40 -23548476/1713 mailto: shantha@hd 1. vsnl. net. ininfo@shant habiotech. com 27 © Copyright Shantha Biotechnics Ltd. , 2000 All rights reserved
- Slides: 27