Shampoos 1 2 The primary function of shampoo



















- Slides: 32

Shampoos 1

2

The primary function of shampoo is to clean hair and scalp. v THE TERM: The word comes from the Hindi “to press”. Originally used in English for “massaging” It still describes part of process of a Turkish bath. v The first shampoos contained soaps (Sodium, potassium and ammonium salts of oleic and coconut fatty acids), but these days they are based on non-soap detergent (synthetic detergents have replaced soaps as the primary surfactant in shampoos because of their superior performance in hard water). 3

v The first large-scale development of non-soap detergent took place in Germany during the First World War (because fats were in short supply) v The technology of complex shampoo emulsion has also become more important as a result of the development of effective two in one shampoo, which clean and condition hair at the same time. 4

A WELL FORMULATED SHAMPOO 1. Should easily spread over the hair. 2. Produce a rich, creamy lather and stable foam which easily rinsed out with water. 3. After drying, the hair should be left in lustrous condition and easily manageable. 4. Surfactant level must be relatively high 10 -20%. 5. Viscosity at least 2000 cps 6. Must exhibit low skin and eye irritation. 5

First shampoos (soaps) have several disadvantages 1. If they used with hard water or low p. H, they leave the hair with dull, tacky coating. 2. Soap solutions are alkaline, causing roughening of scales of the cuticle, giving rise to dullness and fly effect. (Amine soap gives neutral solutions). ٭ Nowadays, most shampoos are based on synthetic detergent. 6

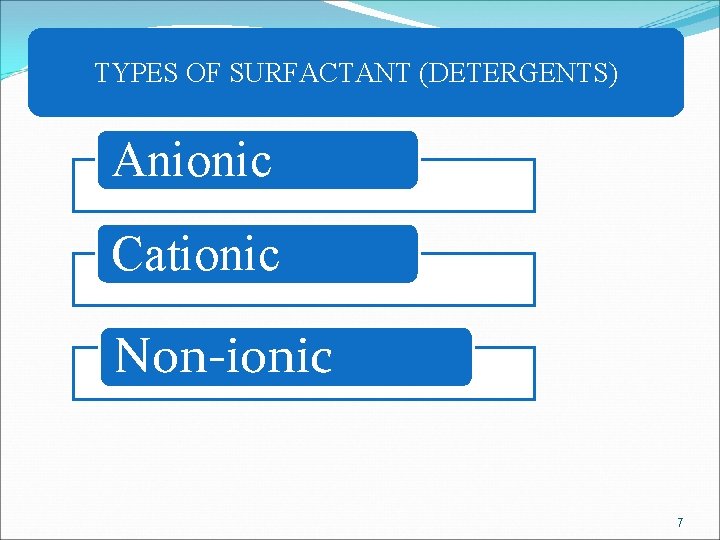
TYPES OF SURFACTANT (DETERGENTS) Anionic Cationic Non-ionic 7

Anionic The alkyl and alkyl ether sulfate represent two most widely used classes of surfactant for the formulation of shampoos. 1. Alkyl sulphates C 12 H 25 O. SO 3 Na+ → SLS C 12 H 25 O. SO 3 HN(CH 2 OH)3 → TLS They are usually mixture, C 12 lauryl, C 14 myristyl, C 16 cetyl, C 18 stearyl. Coconut oil contains 50% each of C 12 and C 14. Lauryl produces the most lather and myristyl the richest lather. ٭ alkyl sulphate clean the hair well, and leave it shiny, but they tend to strip the hair, leaving it unmanageable. 8

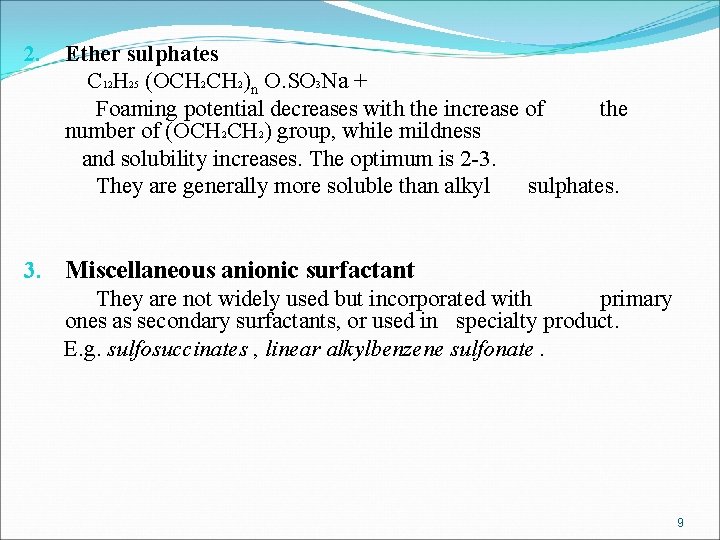
2. Ether sulphates C 12 H 25 (OCH 2)n O. SO 3 Na + Foaming potential decreases with the increase of the number of (OCH 2) group, while mildness and solubility increases. The optimum is 2 -3. They are generally more soluble than alkyl sulphates. 3. Miscellaneous anionic surfactant They are not widely used but incorporated with primary ones as secondary surfactants, or used in specialty product. E. g. sulfosuccinates , linear alkylbenzene sulfonate. 9

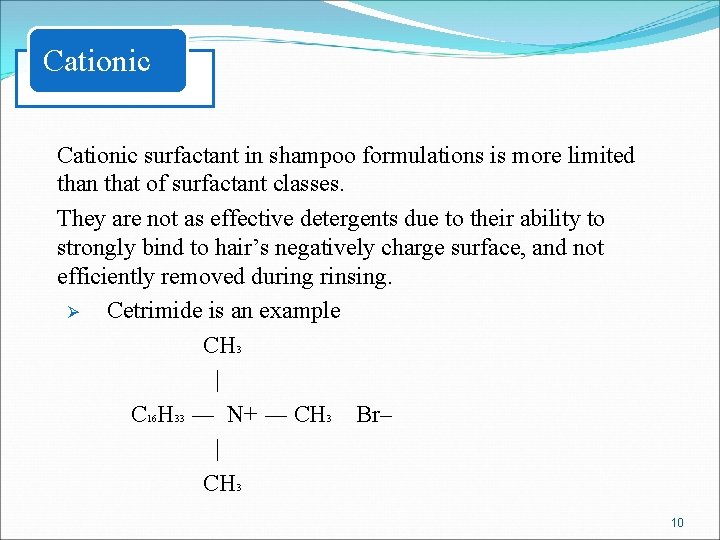
Cationic surfactant in shampoo formulations is more limited than that of surfactant classes. They are not as effective detergents due to their ability to strongly bind to hair’s negatively charge surface, and not efficiently removed during rinsing. Ø Cetrimide is an example CH 3 | C 16 H 33 ― N+ ― CH 3 Br– | CH 3 10

Notes Shampoos containing only one of the previous types of detergents tends to be harsh, and it’s therefore necessary to add an auxiliary detergent, usually weak detergents which: 1. Increase the viscosity of the shampoo. 2. Stabilize the foam. 3. Have conditioning effect on the hair. 4. Increase the solubility of the primary detergent.

Nonionic For example, polyoxyehtylene lauryl ether C 12 H 25 (OCH 2)23 v THE MAIN THREE TYPES OF DETERGENTS: NON-IONIC 1. Fatty alkanolamaides ( The ethanol amides) They work as foam and viscosity enhancers. The main component is the uncharged nonionic amide. The major concern in formulating with alkanolamides is the potential for free amine, particularly diethanolamine (DEA), to form carcinogenic n-nitrosamines. E. g. lauric diethanolamide O ║ CH 2 OH C 11 H 23 C-N CH 2 OH 12

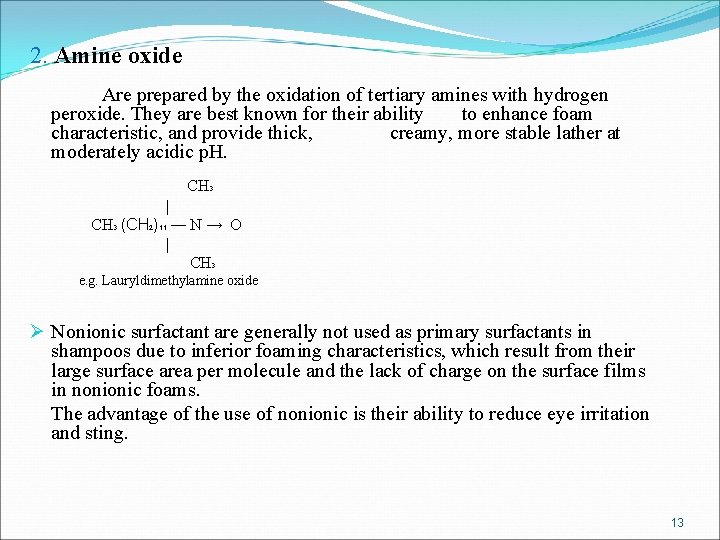
2. Amine oxide Are prepared by the oxidation of tertiary amines with hydrogen peroxide. They are best known for their ability to enhance foam characteristic, and provide thick, creamy, more stable lather at moderately acidic p. H. CH 3 | CH 3 (CH 2)11 ― N → O | CH 3 e. g. Lauryldimethylamine oxide Ø Nonionic surfactant are generally not used as primary surfactants in shampoos due to inferior foaming characteristics, which result from their large surface area per molecule and the lack of charge on the surface films in nonionic foams. The advantage of the use of nonionic is their ability to reduce eye irritation and sting. 13

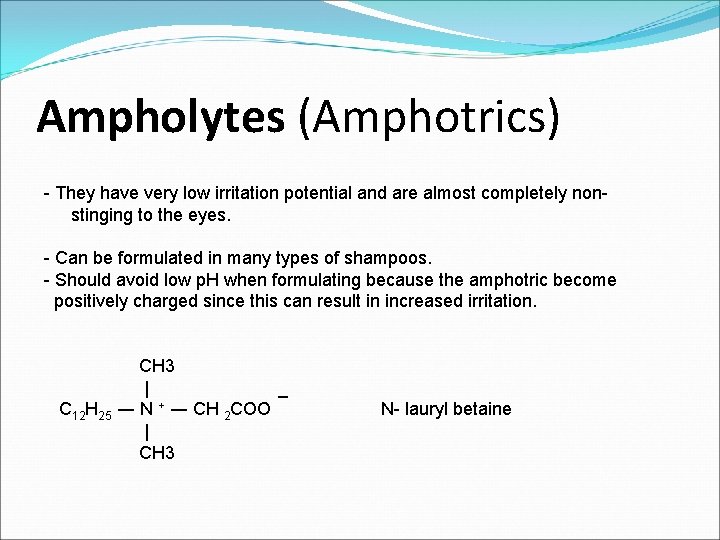
Ampholytes (Amphotrics) - They have very low irritation potential and are almost completely nonstinging to the eyes. - Can be formulated in many types of shampoos. - Should avoid low p. H when formulating because the amphotric become positively charged since this can result in increased irritation. CH 3 | _ C 12 H 25 ― N + ― CH 2 COO N- lauryl betaine | CH 3

Other Additives Many additives associated with shampoos are added to provide various additional benefits to the product; some are critical and others are optional. 1. Viscosity modifiers - Auxiliary detergents and conditioning polymers can provide a thickening effect to a shampoo. - Salts increase viscosity of detergent solutions, Na. Cl 1 -2%, Ammonium chloride at p. H well below 7 to avoid liberation of ammonia, salting out mechanism (bell-shaped curve). - Cellulose derivatives (0. 5 -1. 5%) 2. Weak organic acids A shampoo having the same p. H as hair would do the least damage. ٭ Hair p. H = 5 -6 , S. Detergent p. H = 6 -8 An addition of citric acid will decrease p. H to optimum p. H 5. 5 15

3. Opacifiers The opacifying agent are usually stearates (stearic acid, magnesium stearate, ethylene glycol monostearate), are used in less than 1% concentration to give creamy appearance. 4. Antioxidants/ Sequestrants / UV Absorber Ø Antioxidants are included in shampoo formulation to avoid oxidation of unsaturated components, such as vegetable oil. Ø Sequestering agent is included in shampoo to prevent discoloration and to improve the performance of the anti microbial agents by forming soluble complex with metal ions. Ø Shampoo in clear packaging should contain a UV absorber, e. g. benzophenone-4, to protect against color fading and other reasons upon prolonged exposure to light. 5. Preservatives Are essential component of a shampoo formulation to protect against microbial growth. E. g. parabens. 16

Effect of shampoo on hair Shampoos are also involved in damage of hair, either directly; through removal of structural components of the hair fibers, of indirectly; through removal of protective deposits on the hair. 1. Direct damage: Studies indicated that the nonkeratinious regions of the hair, which include the endocuticle, or inner portion of the cuticle, and the cell membrane complex are susceptible to damage by surfactant molecules. Exposure to shampoos can have deleterious effect on hair structure over time. 2. Indirect damage: It is a result of fiber abrasion occurring when hairs are rubbed against each other during cleaning. More important is the removal of sebum from the fiber surface during shampooing (sebum act as natural lubricant for hair). 17

Shampoo benefits Shampoo formulations are classified into four basic functions: Cleaning, mild (or baby), conditioning, and antidandruff. ٭ Mild or baby shampoos is required to allow only minimal eye, scalp, and skin irritant. They often contain mild surfactant systems, such as nonionics and amphotrics. 18

Examples of shampoo’s formulas Cleansing shampoo Ingredient % Ammonium lauryl sulfate 15 Cocamide DEA 2 Cocamidopropyl betain 2 Fragrance 0. 7 Preservative 0. 5 Citric acid 0. 3 Ammonium chloride 0. 2 Color 0. 001 Water q. s 19

Baby shampoo Ingredient % PEG-80 sorbitan laurate 12 Sodium trideceth sulfate 5 Sodium lauroamphoacetate 5 PEG-120 methyl glucose dioleate 2 Cocamidopropyl hydroxysultaine 1 Fragrance 0. 7 Preservative 0. 5 Color 0. 01 Water q. s 20

Conditioners 21

Does hair need conditioner? Conditioners are formulated to impart conditioning to hair, for example; improve combing, softness, and manageability, in addition to the usual cleaning benefits. Physical damaging effect: ♦ Wind, weather, ultraviolet radiation, brushing and combing. Chemical damaging effects: 1. Shampoos which remove natural lipids 2. Dyeing require preliminary bleaching 3. Bleaching with alkaline hydrogen peroxide Drastic treatment which cause severe weakening of the hair fibers and loss of elasticity 4. Permanent waving Where peroxide and thioglycollate attack the disulphide and peptide bonds of keratin. 22

Ø Conditioning term is difficult to define The process of conditioning involves at least four functions: 1. 2. 3. 4. Rendering the hair manageable, easy to comb and set. Preserve its natural appearance and luster. Exerting a softening effect. Giving “BODY” to the hair. 23

Conditioners ingredients Emollients Compounds containing positively charged nitrogen Acids Proteins 24

Emollients Oil adheres to hair fibers → body + luster + lubrication Excessive greasiness can be overcome by applying the lipid in the form of diluted O/W emulsion and by using high melting point lipids, such as cetostearyl alcohol. Ø Vegetable oil, lanolin and its soluble derivatives (POE or quaternaries), synthetic esters, sebum substitutes, Octyl stearates and Squalene Ø Silicones have high refractive indices, and are therefore good luster producing compounds. 25

Compounds containing positively charged nitrogen Keratin contains more acid groups than basic groups, and therefore tend to attract positively charged molecules and ions. 1. Cationic compounds (quaternary ammonium compounds), a typical example → cetrimide 2. Amidoamides (tertiary amines). a typical example → stearamidopropyl dimethylamine lactate 3. Alkanolamide, example → Coconut diethanolamides 4. Ampholytes, example → N- alkylbetaines as anti irritant. 5. Polyvinylpyrolidone (PVP). 26

Acids The conditioning effects of weak acids have been known since ancient times (lemon juice, vinegar) 1. They dissolve calcium and magnesium deposits. 2. Neutralize excess alkali after bleaching or permanent waving. 3. Precipitate the soluble keratin products resulted from the rupture of peptide bonds by the action of peroxide or thiglycollate. Ø A suitable lipid mixture is emulsified with cationic surfactant → O/W and a small quantity of weak organic acid and protein can be added. 27

Proteins are actually mixture of: 1. 2. 3. 4. Proteins polypeptides peptide amino acids We can prepare them by hydrolyzing protein usually with enzyme → Collagen, Milk, Oats, Wheat, Soya. 28

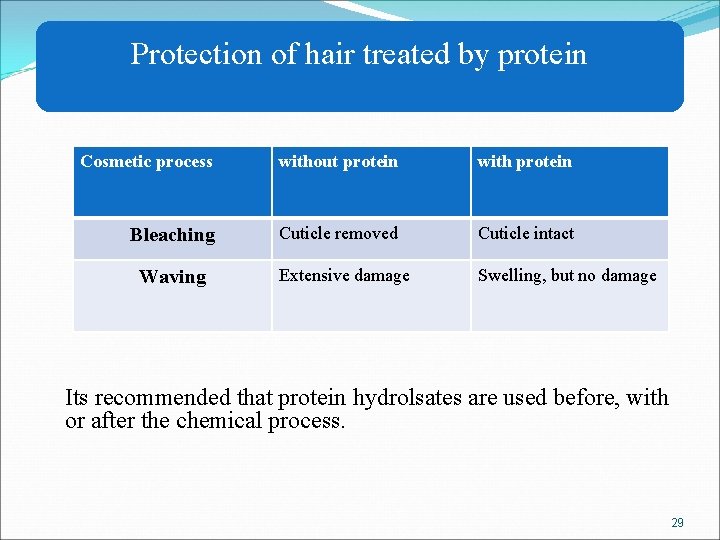
Protection of hair treated by protein Cosmetic process without protein with protein Bleaching Cuticle removed Cuticle intact Extensive damage Swelling, but no damage Waving Its recommended that protein hydrolsates are used before, with or after the chemical process. 29

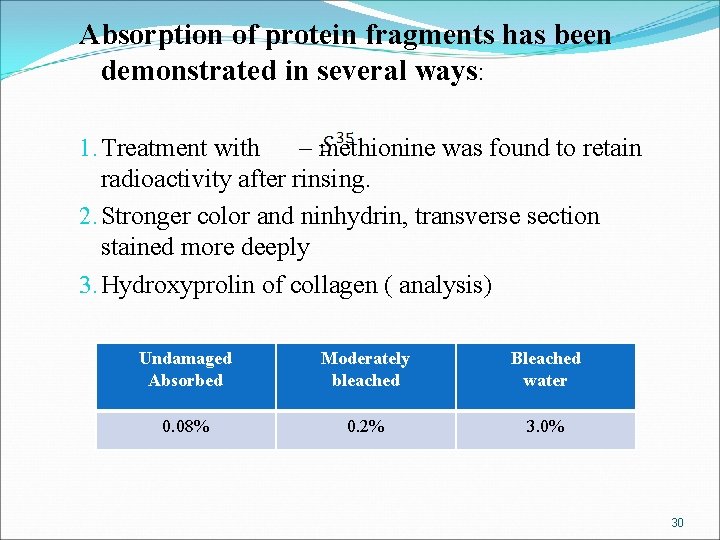
Absorption of protein fragments has been demonstrated in several ways: 1. Treatment with – methionine was found to retain radioactivity after rinsing. 2. Stronger color and ninhydrin, transverse section stained more deeply 3. Hydroxyprolin of collagen ( analysis) Undamaged Absorbed Moderately bleached Bleached water 0. 08% 0. 2% 3. 0% 30

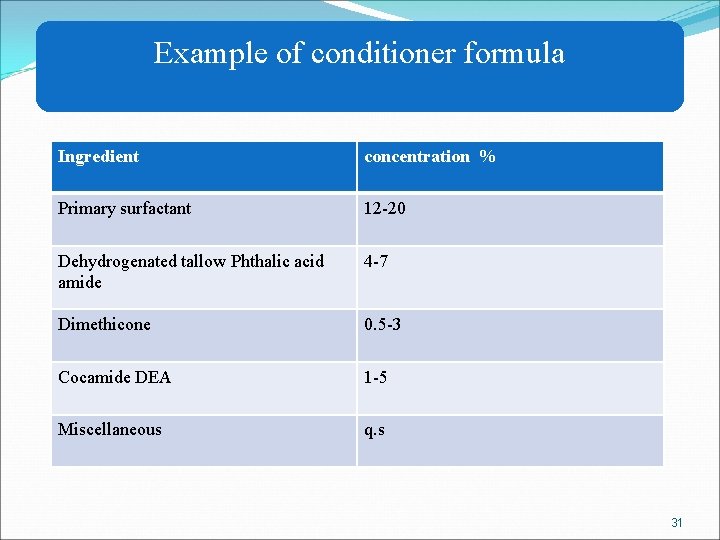
Example of conditioner formula Ingredient concentration % Primary surfactant 12 -20 Dehydrogenated tallow Phthalic acid amide 4 -7 Dimethicone 0. 5 -3 Cocamide DEA 1 -5 Miscellaneous q. s 31

32