Shajara Preparatory School E Lessons Structure of matter

- Slides: 20

Shajara Preparatory School E. Lessons Structure of matter Objectives: . To identify structure of atom. . To identify atomic number of an element. . To identify mass number of an atom. . To identify ions and their different types. . To identify molecule. Grade Eight (A) www. shajara. webnode. com

Shajara Preparatory School E. Lessons Structure of matter What is Atom Table of contents Parts of atom Particles of atom Mass number & atomic number What is ion How is the ion form Grade Eight (A) www. shajara. webnode. com

Shajara Preparatory School E. Lessons Structure of matter Atom Ion Grade Eight (A) www. shajara. webnode. com Molecule

Shajara Preparatory School E. Lessons Matter is made up of tiny particles called atom. The atom has a spherical shape. Watch Video www. shajara. webnode. com

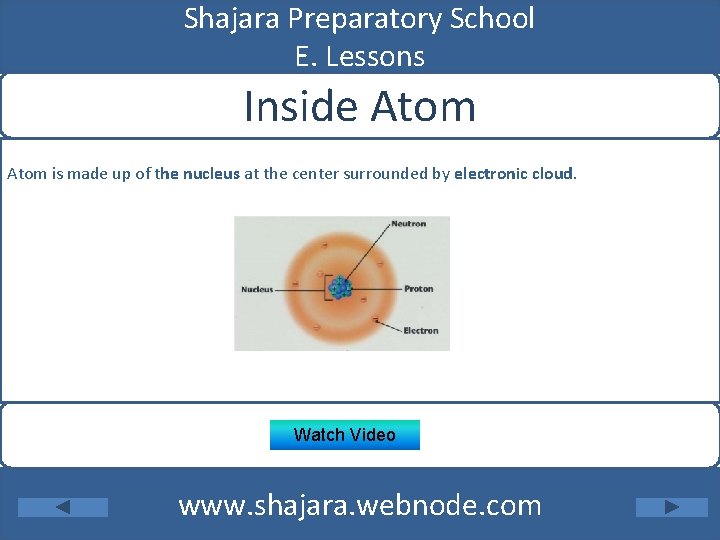
Shajara Preparatory School E. Lessons Inside Atom is made up of the nucleus at the center surrounded by electronic cloud. Watch Video www. shajara. webnode. com

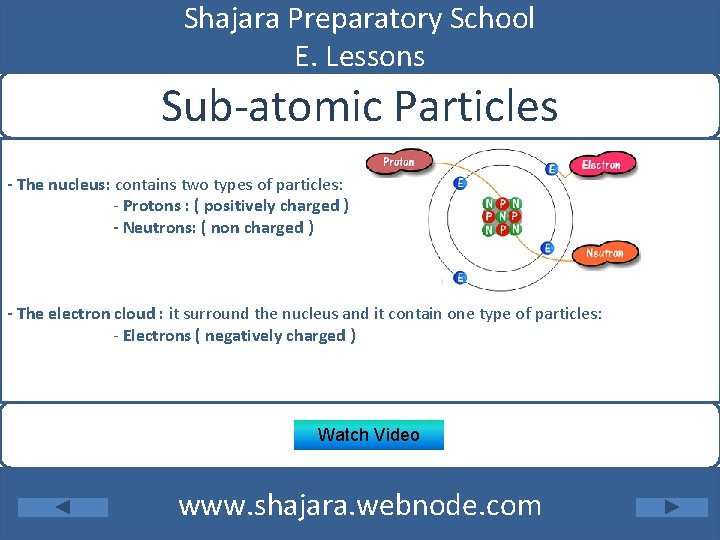
Shajara Preparatory School E. Lessons Sub-atomic Particles - The nucleus: contains two types of particles: - Protons : ( positively charged ) - Neutrons: ( non charged ) - The electron cloud : it surround the nucleus and it contain one type of particles: - Electrons ( negatively charged ) Watch Video www. shajara. webnode. com

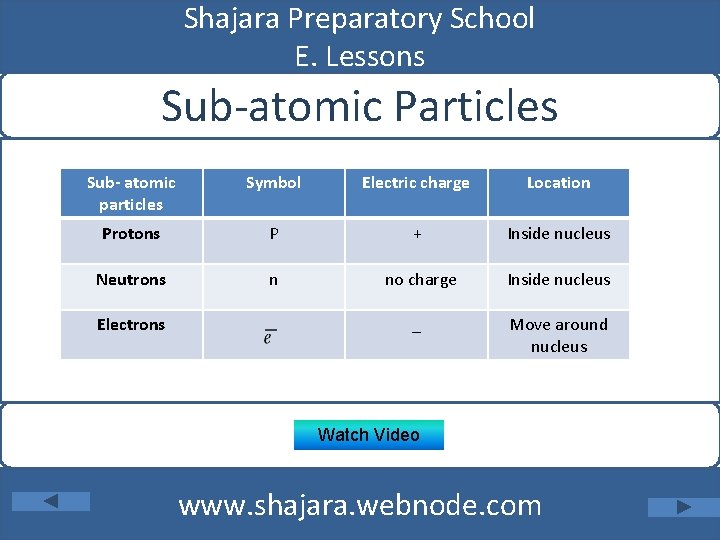
Shajara Preparatory School E. Lessons Sub-atomic Particles Sub- atomic particles Symbol Electric charge Location Protons P + Inside nucleus Neutrons n no charge Inside nucleus _ Move around nucleus Electrons Watch Video www. shajara. webnode. com

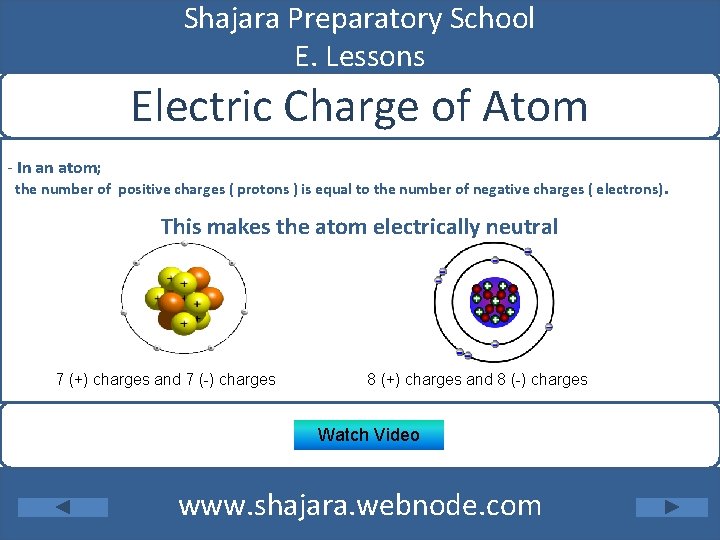
Shajara Preparatory School E. Lessons Electric Charge of Atom - In an atom; the number of positive charges ( protons ) is equal to the number of negative charges ( electrons). This makes the atom electrically neutral 7 (+) charges and 7 (-) charges 8 (+) charges and 8 (-) charges Watch Video www. shajara. webnode. com

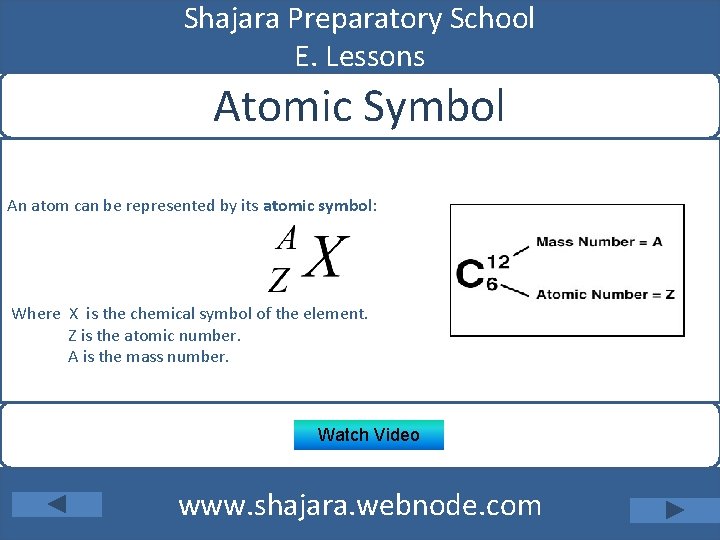
Shajara Preparatory School E. Lessons Atomic Symbol An atom can be represented by its atomic symbol: Where X is the chemical symbol of the element. Z is the atomic number. A is the mass number. Watch Video www. shajara. webnode. com

Shajara Preparatory School E. Lessons Atomic number= number of protons Z=P For example, The element carbon has 6 protons in its nucleus. Then, Its atomic number Z=6. Watch Video www. shajara. webnode. com

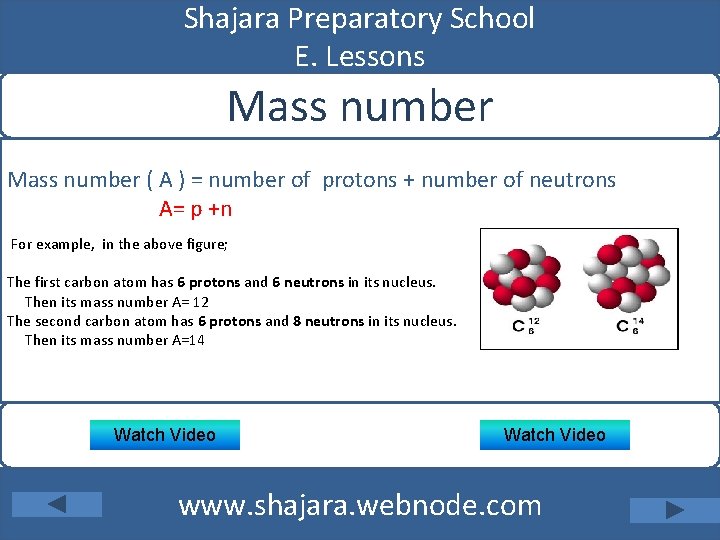
Shajara Preparatory School E. Lessons Mass number ( A ) = number of protons + number of neutrons A= p +n For example, in the above figure; The first carbon atom has 6 protons and 6 neutrons in its nucleus. Then its mass number A= 12 The second carbon atom has 6 protons and 8 neutrons in its nucleus. Then its mass number A=14 Watch Video www. shajara. webnode. com

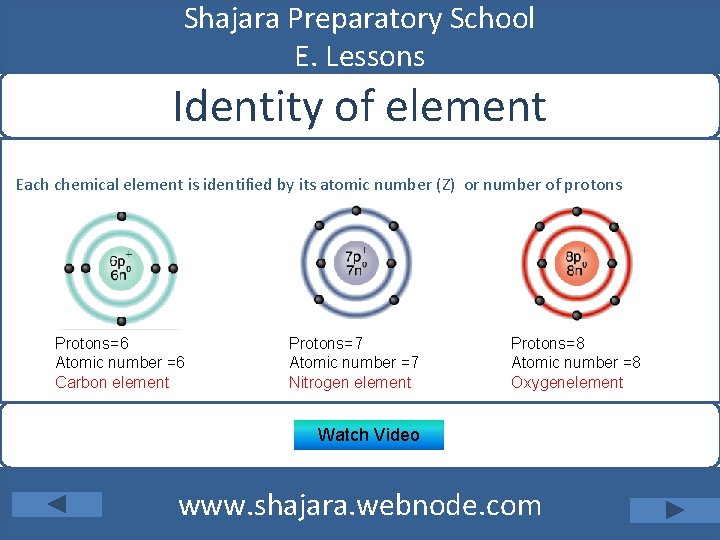
Shajara Preparatory School E. Lessons Identity of element Each chemical element is identified by its atomic number (Z) or number of protons Protons=6 Atomic number =6 Carbon element Protons=7 Atomic number =7 Nitrogen element Protons=8 Atomic number =8 Oxygenelement Watch Video www. shajara. webnode. com

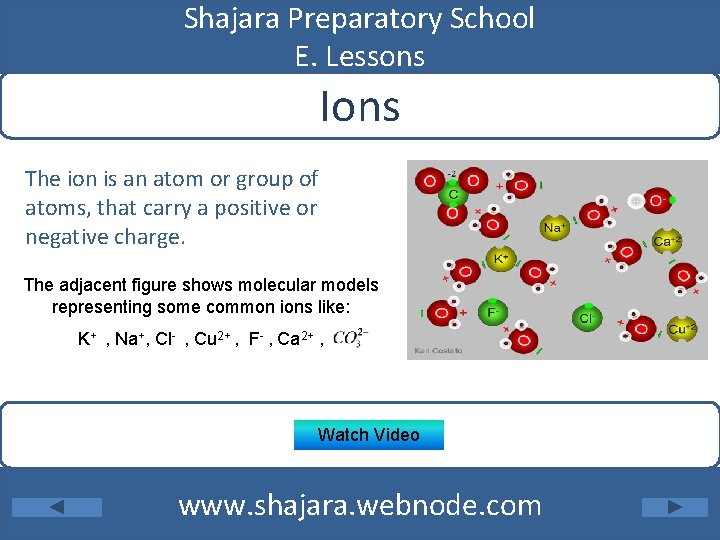
Shajara Preparatory School E. Lessons Ions The ion is an atom or group of atoms, that carry a positive or negative charge. The adjacent figure shows molecular models representing some common ions like: K+ , Na+, Cl- , Cu 2+ , F- , Ca 2+ , Watch Video www. shajara. webnode. com

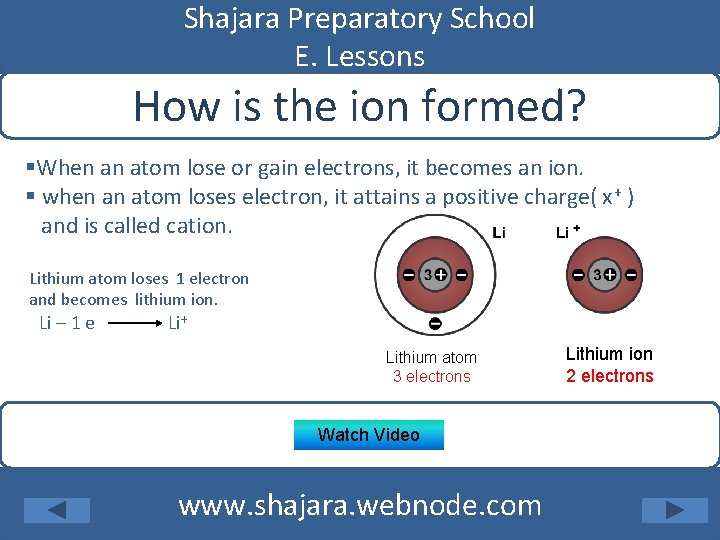
Shajara Preparatory School E. Lessons How is the ion formed? §When an atom lose or gain electrons, it becomes an ion. § when an atom loses electron, it attains a positive charge( x+ ) and is called cation. Lithium atom loses 1 electron and becomes lithium ion. Li – 1 e Li+ Lithium atom 3 electrons Watch Video www. shajara. webnode. com Lithium ion 2 electrons

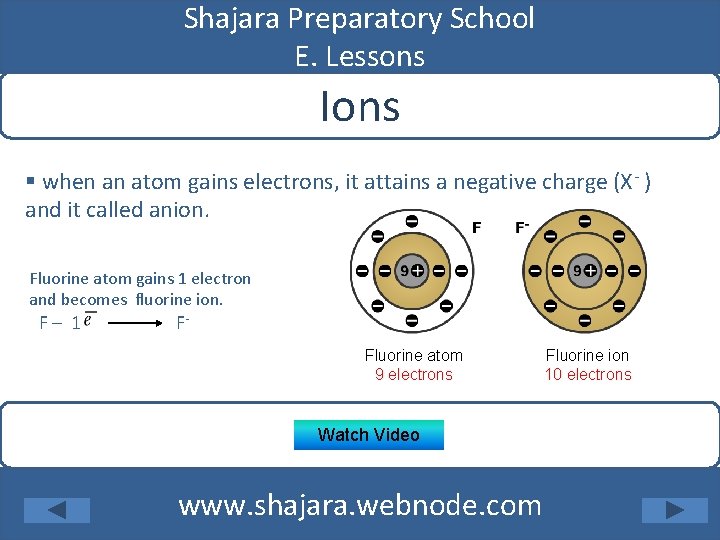
Shajara Preparatory School E. Lessons Ions § when an atom gains electrons, it attains a negative charge (X- ) and it called anion. Fluorine atom gains 1 electron and becomes fluorine ion. F– 1 F- Fluorine atom 9 electrons Watch Video www. shajara. webnode. com Fluorine ion 10 electrons

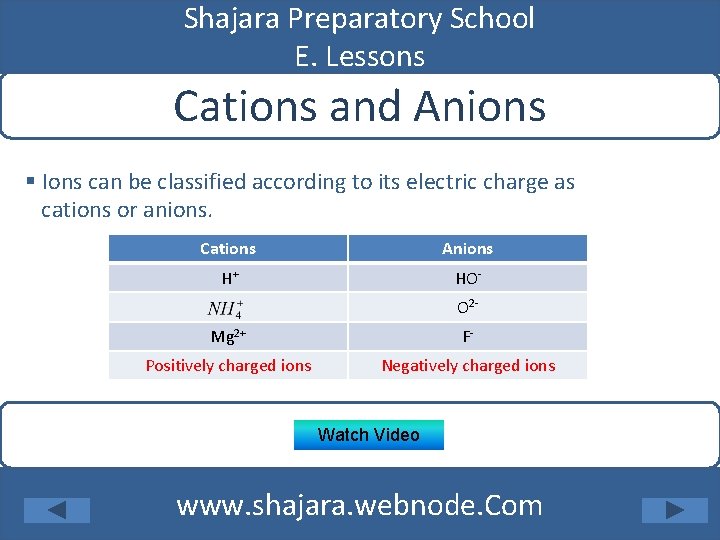
Shajara Preparatory School E. Lessons Cations and Anions § Ions can be classified according to its electric charge as cations or anions. Cations Anions H+ HOO 2 - Mg 2+ F- Positively charged ions Negatively charged ions Watch Video www. shajara. webnode. Com

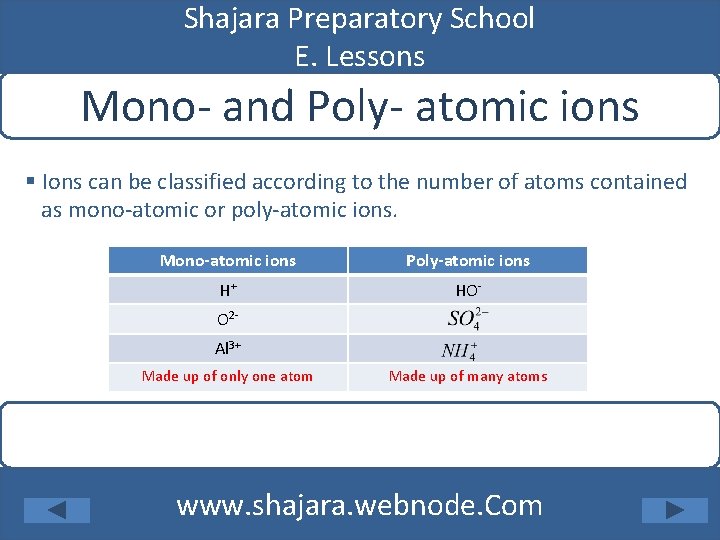
Shajara Preparatory School E. Lessons Mono- and Poly- atomic ions § Ions can be classified according to the number of atoms contained as mono-atomic or poly-atomic ions. Mono-atomic ions Poly-atomic ions H+ HO- O 2 Al 3+ Made up of only one atom Made up of many atoms www. shajara. webnode. Com

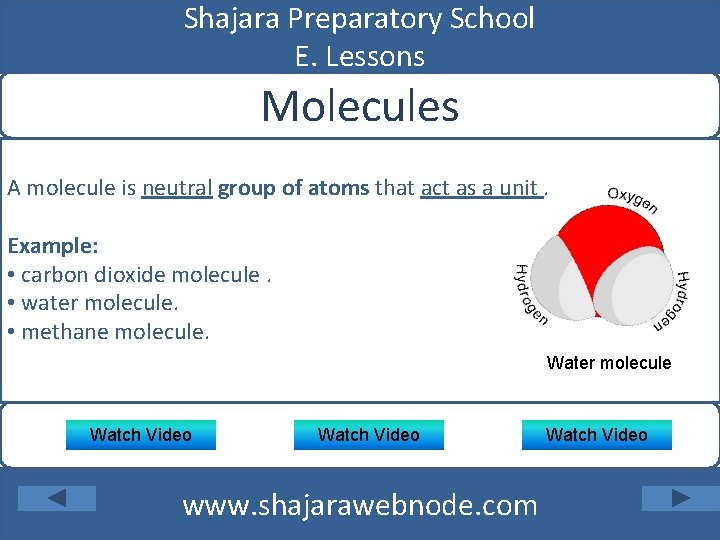
Shajara Preparatory School E. Lessons Molecules A molecule is neutral group of atoms that act as a unit. Example: • carbon dioxide molecule. • water molecule. • methane molecule. Water molecule Watch Video www. shajarawebnode. com Watch Video

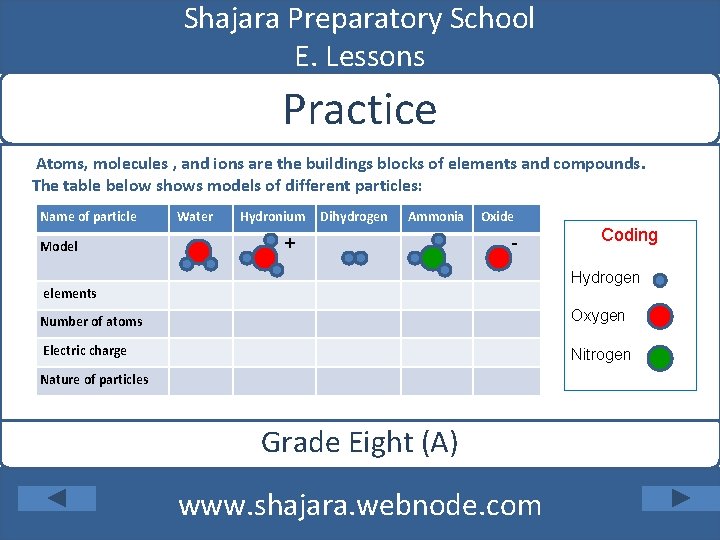
Shajara Preparatory School E. Lessons Practice Atoms, molecules , and ions are the buildings blocks of elements and compounds. The table below shows models of different particles: Name of particle Model Water Hydronium Dihydrogen Ammonia + Oxide - Coding Hydrogen elements Number of atoms Oxygen Electric charge Nitrogen Nature of particles Grade Eight (A) www. shajara. webnode. com

Shajara Preparatory School E. Lessons Structure of Matter Prepared by: Firas Sami Al Haj Moussa Wissam Saddiq Basel Fares Ahmad manasri Hassan shawky Supervised by: T. Wissam Abdallah Grade Eight (A) Students www. shajara. webnode. com