Sexually Transmitted Infections STI AND Expedited Partner Treatment


















- Slides: 30

Sexually Transmitted Infections (STI) AND Expedited Partner Treatment (EPT) Mayuri Dasari M. D. Cook County Loyola Provident Family Medicine Residency Program

GOALS Discuss and Educate health care providers on Sexually Transmitted Infections (STI) and Expedited Partner Treatment (EPT), to decrease the rate of reinfection in the community

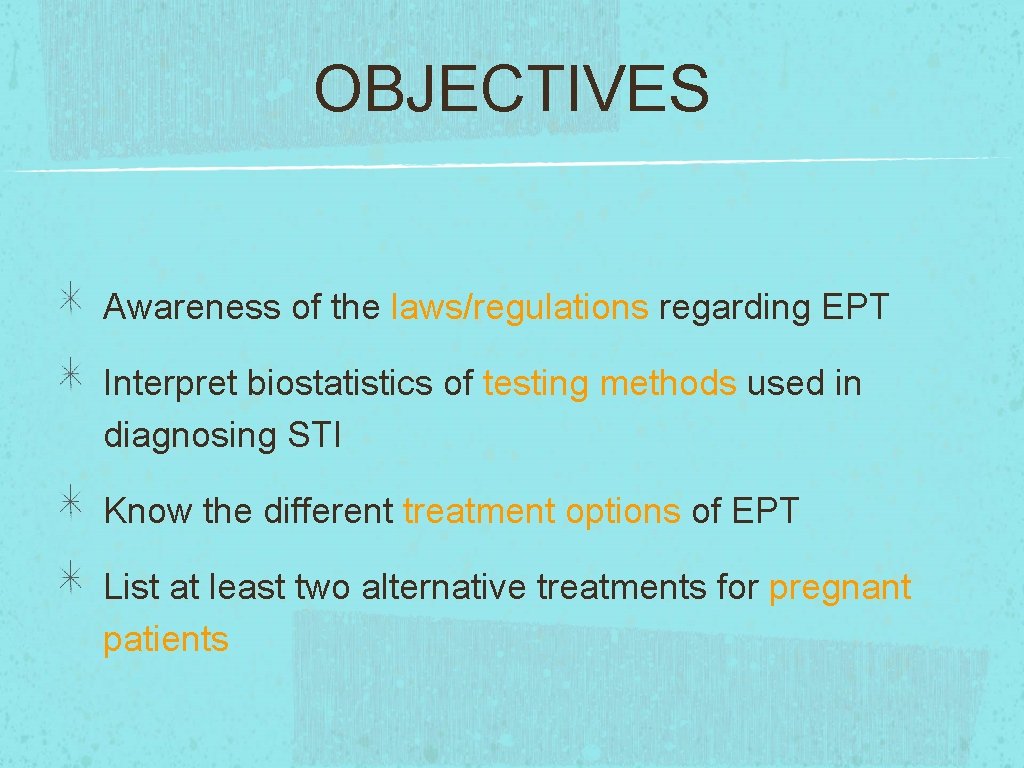
OBJECTIVES Awareness of the laws/regulations regarding EPT Interpret biostatistics of testing methods used in diagnosing STI Know the different treatment options of EPT List at least two alternative treatments for pregnant patients

Expedited Partner Treatment (EPT) It is the practice of treating partners of persons with STI without an intervening medical evaluation or professional prevention counseling. Implementation of EPT is primarily through patient delivered partner treatment (PDPT) RCT funded by CDC revealed decrease in reinfection rates with EPT compared to other methods

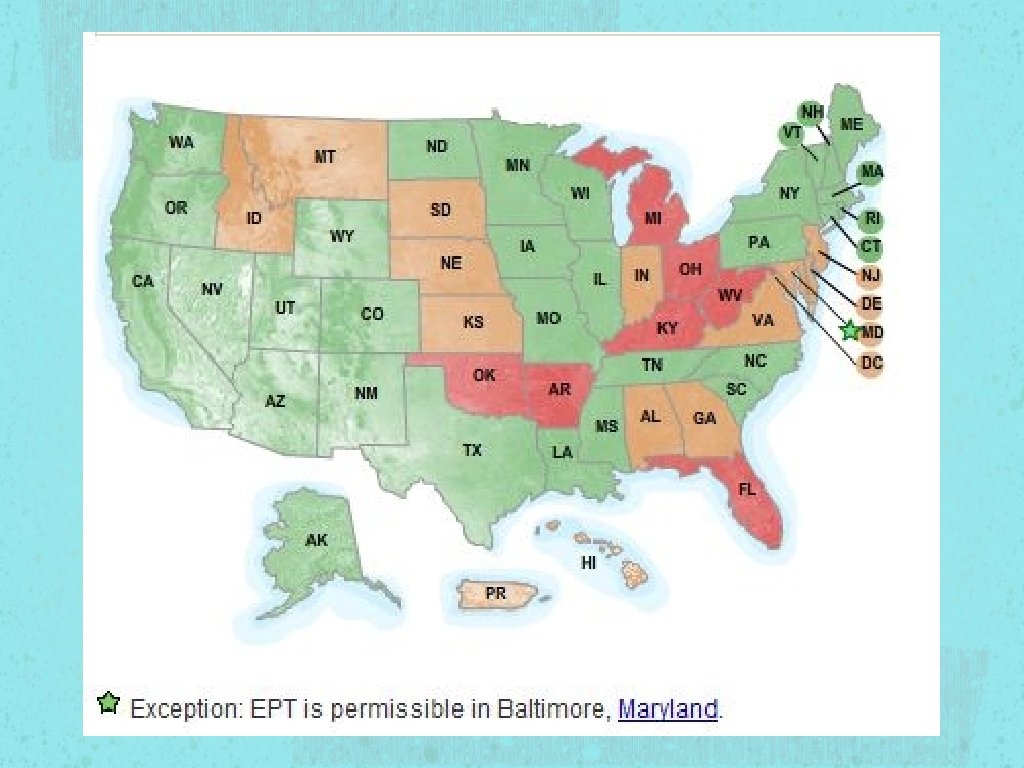
Laws regarding EPT is permissible in 32 states EPT is potentially allowable in 11 states EPT is prohibited in 7 states See Handout For details


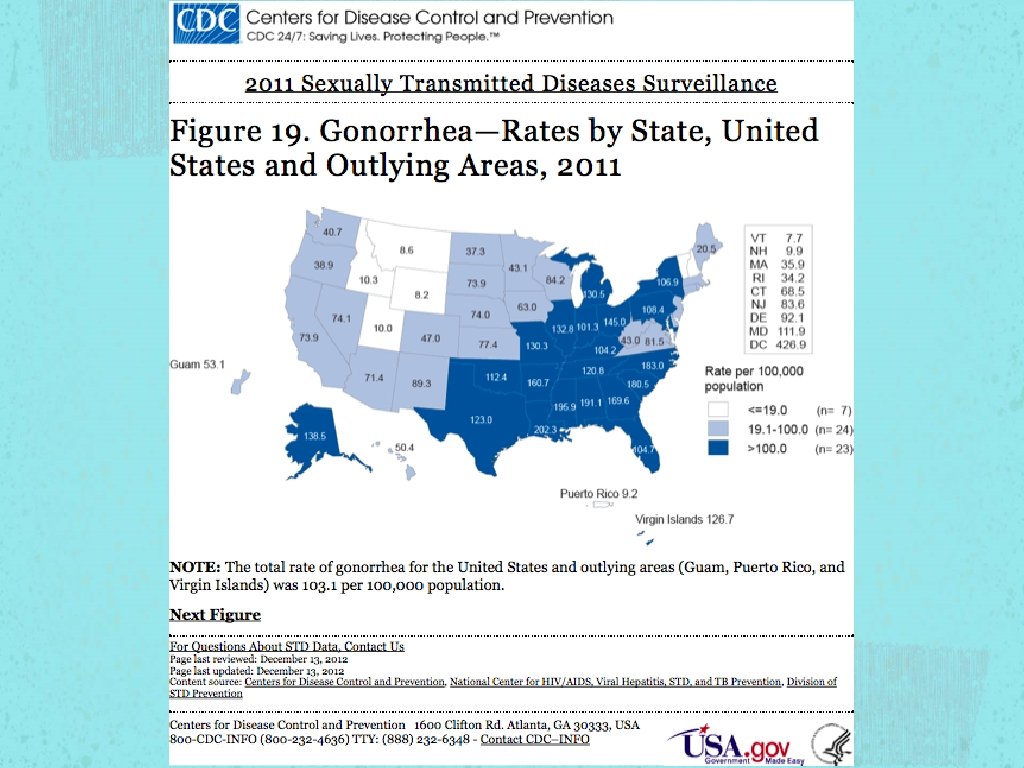
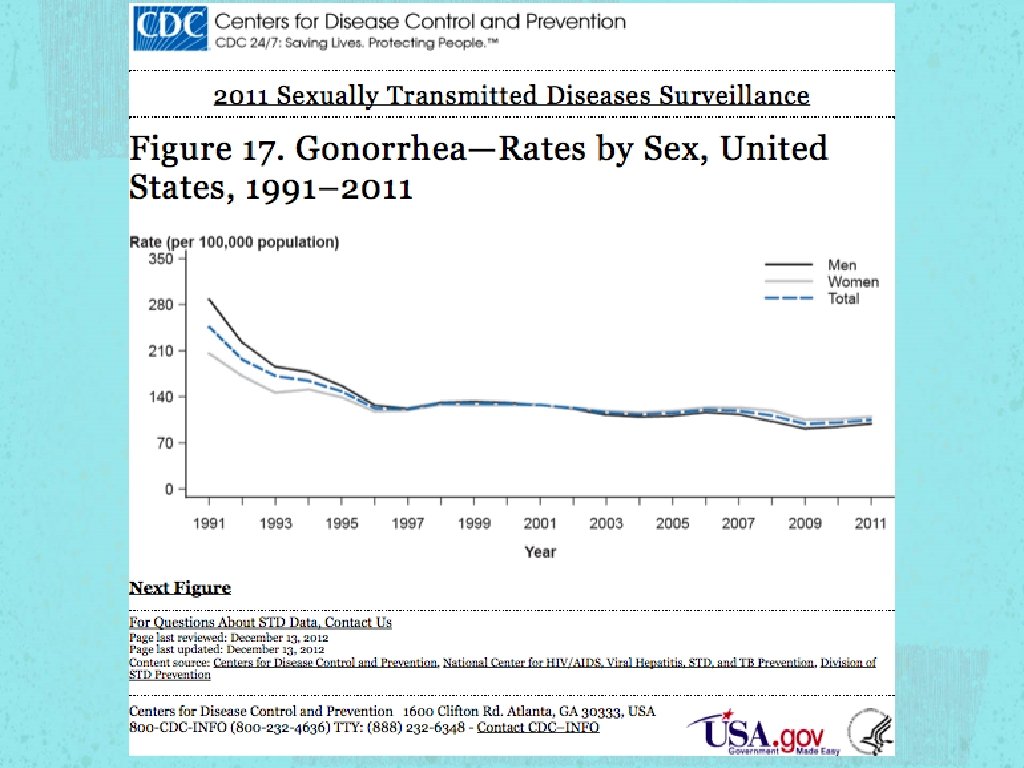
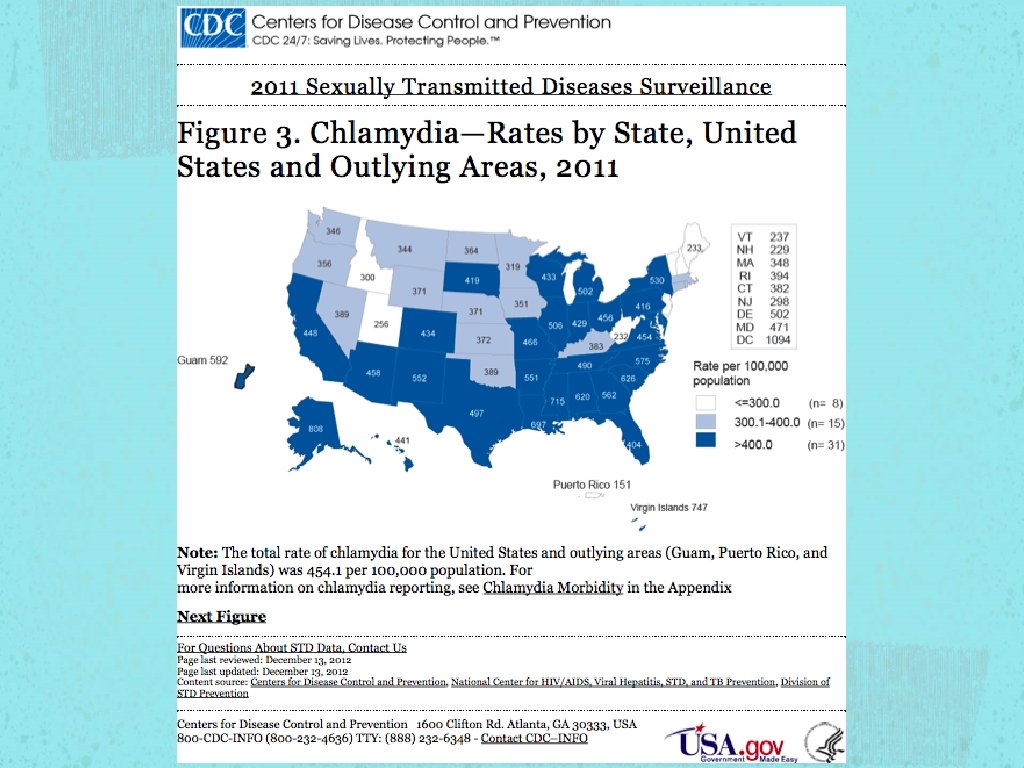
STI According to 2010 CDC estimations, the most troubling trends were seen in the three most treatable STI in the US Gonorrhea Chlamydia Syphilis

STI Trichomoniasis Symptoms more prominent in women compared to men Increased risk of SGA and premature delivery

Gonorrhea Constant Fluctuation of prevalence rate Susceptibility Ciprofloxacin - 6% - 8% from 2009 to 2011 Azithroymycin - MSM (EPT not recommended in MSM)



Chlamydia The most commonly reported notifiable disease in the United States, per the CDC Highest prevalence by sex, in young women (especially age 15 - 24), which could be a reflection of screening recommendations. Highest prevalence by race is seen in African. Americans



Trichomoniasis Trend data for this infection are limited to estimates of initial physician visits from the NDTI (National Disease and Therapeutic Index) The highest prevalence is noticed among blacks at 13% per CDC The overall prevalence in 2004 was 3%


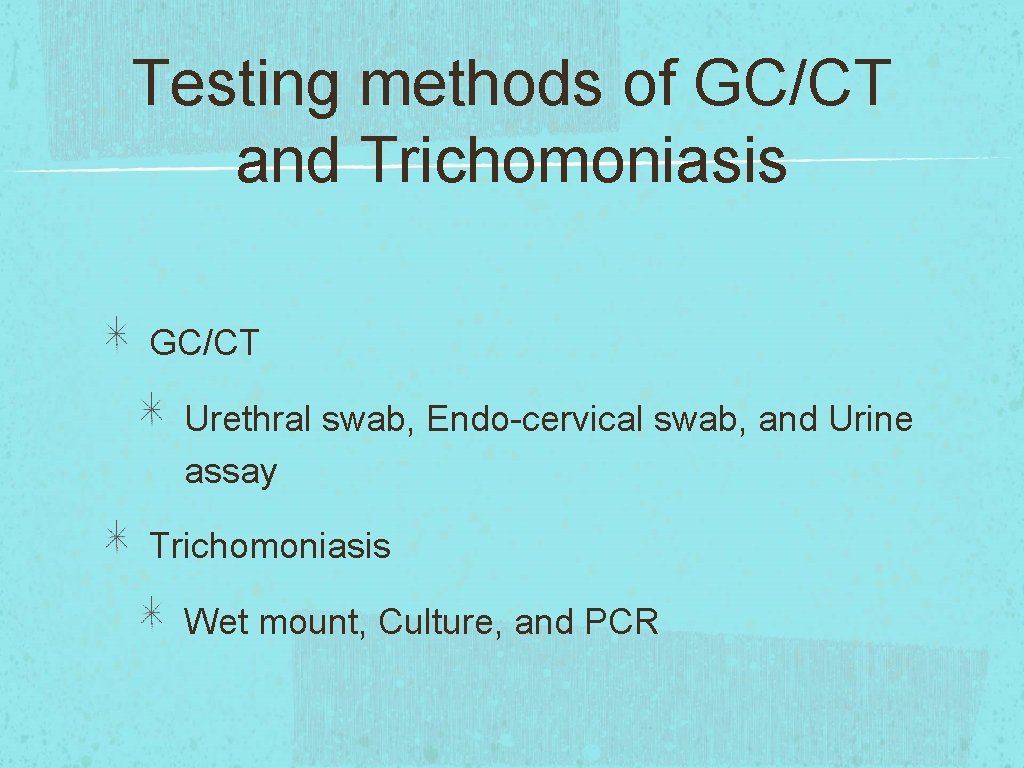
Testing methods of GC/CT and Trichomoniasis GC/CT Urethral swab, Endo-cervical swab, and Urine assay Trichomoniasis Wet mount, Culture, and PCR

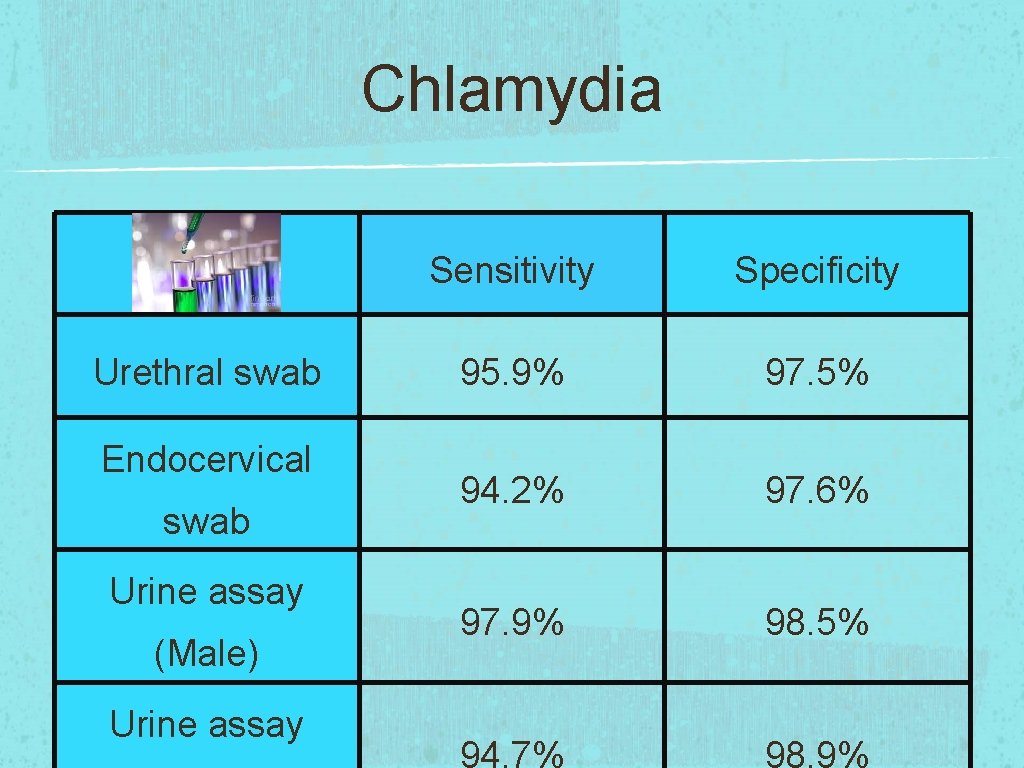
Chlamydia Urethral swab Endocervical swab Urine assay (Male) Urine assay Sensitivity Specificity 95. 9% 97. 5% 94. 2% 97. 6% 97. 9% 98. 5% 94. 7% 98. 9%

Gonorrhea Urethral swab Endocervical swab Urine assay (Male) Urine assay Sensitivity Specificity 99. 1% 97. 8% 99. 2% 98. 7% 98. 5% 99. 6% 91. 3% 99. 3%

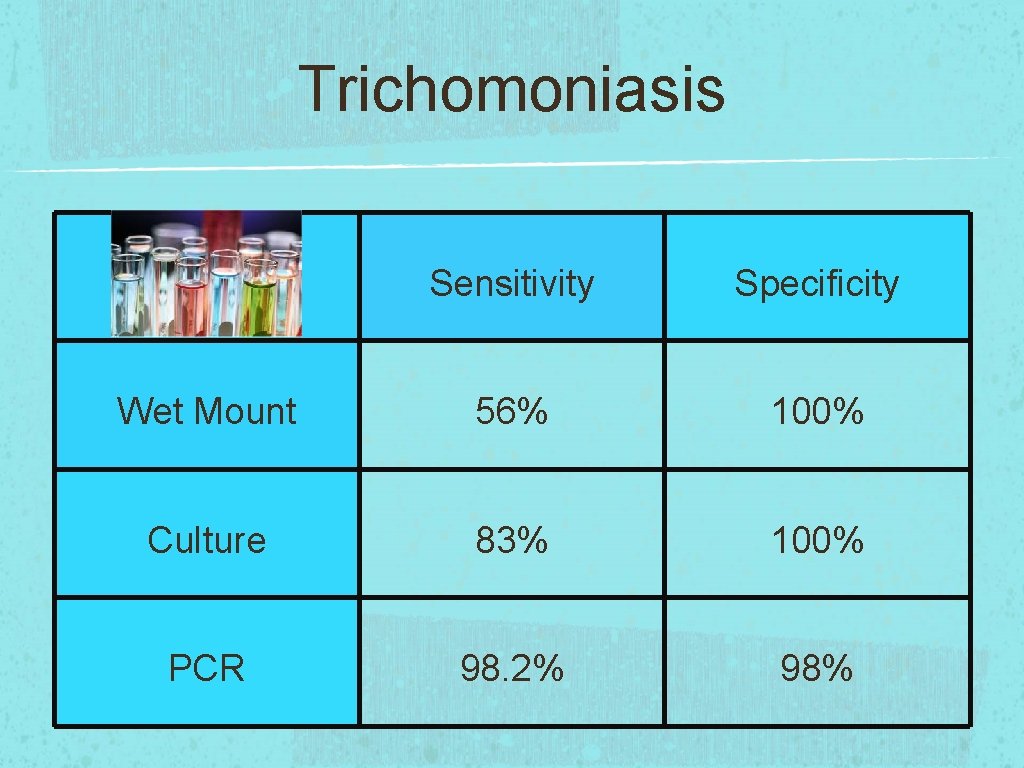
Trichomoniasis Sensitivity Specificity Wet Mount 56% 100% Culture 83% 100% PCR 98. 2% 98%

Treatment Options for EPT Chlamydia Gonorrhea Trichomoniasis* *Trichomoniasis is not part of EPT, per CDC

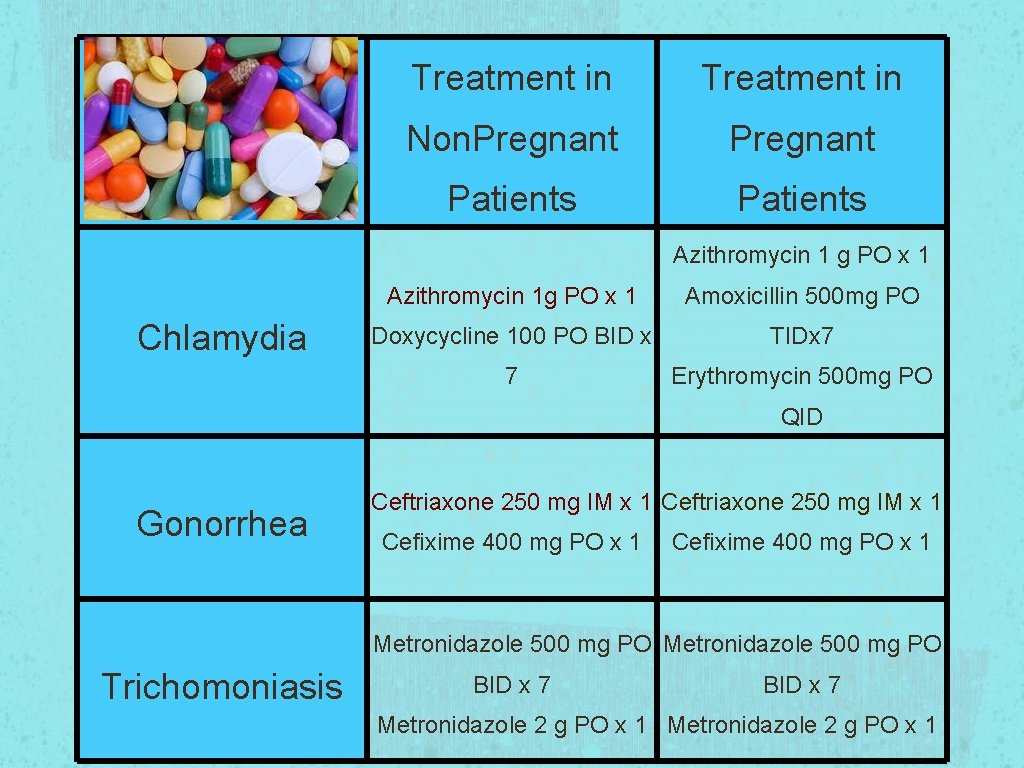
Treatment in Non. Pregnant Patients Azithromycin 1 g PO x 1 Chlamydia Azithromycin 1 g PO x 1 Amoxicillin 500 mg PO Doxycycline 100 PO BID x TIDx 7 7 Erythromycin 500 mg PO QID Gonorrhea Ceftriaxone 250 mg IM x 1 Cefixime 400 mg PO x 1 Metronidazole 500 mg PO Trichomoniasis BID x 7 Metronidazole 2 g PO x 1

Pregnancy Trichomoniasis Delaying treatment in pregnancy is recommended until after first trimester * (per ACOG Guidelines Vs Expert) CDC recommends treating pregnant women regardless of their gestational age.

Re-Testing in Pregnancy CDC recommends testing for re-infection within 3 to 4 weeks after completion of treatment Patient’s sex partners from the preceding 60 days need to be promptly evaluated and treated ACOG recommends following CDC guidelines for treatments and test of cure Abstinence until 7 days after completion of treatment of patient and partner

Re-Testing in Non. Pregnant Patients Gonorrhea Re-test in 1 week all patients treated with alternative treatment methods Increase in resistance to alternative oral treatment

Summary Trend of increase in prevalence of GC/CT infections EPT is permissible in 32 states including Illinois Sensitivity and Specificity is higher with Urine PCR Primary treatment options for common STIs Alternative treatment options are available for Chlamydia and Gonorrhea in pregnant and non pregnant patients Re-testing for Gonorrhea and Chlamydia in pregnant and non pregnant patients

Small Group Discussion

Case 1 - Discussion Urine Hcg Ceftriaxone 250 mg IM x 1 AND Azithromycin 1 g PO x 1 AND Cefixime 400 mg PO x 1 Retest partner in 1 week due to increased resistance to oral treatments for Gonorrhea Safe sex education, abstinence until 7 days after completion of treatment

Case 2 - Discussion Prenatal labs, ultrasound, counseling Azithromycin 1 g PO x 1, OR Amoxicillin 500 mg PO TID x 7 days, OR Erythromycin 500 mg PO QID x 7 days AND Ceftriaxone 250 mg IM x 1 or Cefixime 400 mg PO x 1 Partner - Azithromycin 1 g PO x 1 AND Cefixime 400 mg PO x 1 Test of cure for patient in 3 to 4 weeks Retest partner 1 week after treatment completion

Case 3 - Discussion HIV, RPR, Hep B, Hep C Azithromycin 1 g PO x 1 Cannot give partner treatment because she lives in a state where EPT is prohibited No need for retesting the patient if he received Ceftriaxone IM
 A bacterial std that usually affects mucous membranes
A bacterial std that usually affects mucous membranes Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Chapter 24 lesson 1 sexually transmitted diseases
Chapter 24 lesson 1 sexually transmitted diseases Std
Std Sexually transmitted diseases
Sexually transmitted diseases Std
Std Expedited cost
Expedited cost Normal vs expedited costs
Normal vs expedited costs Understanding the mirai botnet
Understanding the mirai botnet Bone and joint infections
Bone and joint infections Methotrexate and yeast infections
Methotrexate and yeast infections Retroviruses and opportunistic infections
Retroviruses and opportunistic infections Spongia officinalis
Spongia officinalis Opportunistic infections
Opportunistic infections Opportunistic infections
Opportunistic infections Storch infections
Storch infections Storch infections
Storch infections Infections opportunistes digestives
Infections opportunistes digestives Eye infections
Eye infections Postpartum infections
Postpartum infections Genital infections
Genital infections Genital infections
Genital infections Ciliary escalator
Ciliary escalator Acute gingival infections
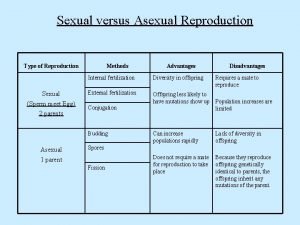
Acute gingival infections How do whales reproduce sexually
How do whales reproduce sexually Is flatworm asexual reproduction
Is flatworm asexual reproduction Parasitism phylum
Parasitism phylum What is sexually dimorphic mean
What is sexually dimorphic mean What is sexually coercive behavior
What is sexually coercive behavior Protists
Protists Was it sexual abuse quiz
Was it sexual abuse quiz