SexGender and Minority Inclusion in NIH Clinical Research
































- Slides: 34

Sex/Gender and Minority Inclusion in NIH Clinical Research What Investigators Need to Know! Presenter: Miriam F. Kelty, Ph. D, National Institute on Aging, 2/2006 1

Overview • Review and Rationale of Policy • Recent Up-Dates & Implications – Definition of Clinical Research – OMB Standards – NIH-Defined Phase III Trials • Resources and Getting Help 2

NIH Policy on Inclusion of Women & Minorities in Clinical Research • Why does NIH have this policy? – Mandated by Congress, 1993 PL 10343 – Ethical principal of justice and importance of balancing research burdens and benefits 3

Public Law PL 103 -43 • Women and Minorities must be included in all clinical research studies • Women and Minorities must be included in Phase III clinical trials & the trial must be designed to permit valid analysis • Cost is NOT allowed as an acceptable reason for exclusion • NIH to support outreach efforts to recruit and retain women, minorities, and their subpopulations in clinical studies 4

NIH Policy on Inclusion • NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research – Amended October, 2001 • http: //grants. nih. gov/grants/funding/ women_min/guidelines_amended_10 _2001. htm 5

Updates to Inclusion Policy • NIH Definition of Clinical Research • New OMB Standards for Data on Ethnicity and Race • Further Clarification about NIHDefined Phase III Clinical Trials 6

NIH Definition of Clinical Research (1) Patient-oriented research. Research conducted with human subjects (or on material of human origin such as tissues, specimens and cognitive phenomena) for which an investigator (or colleague) directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. Patient-oriented research includes: (a) mechanisms of human disease, (b) therapeutic interventions, (c) clinical trials, and (d) development of new technologies; 7

NIH Definition of Clinical Research (continued) (2) Epidemiologic and behavioral studies; (3) Outcomes research and health services research. 8

Update to NIH Policy for Inclusion • New OMB Standards • OMB Directive 15 Issued 1997 – Racial and Ethnic Standards for Federal Statistics and Administrative Reporting – Effective Date No Later Than January 1, 2003 9

Update to NIH Policy for Inclusion • OMB Directive 15 Issued 1997 – Collecting Data by Self-Report: – Two Separate Questions • Question 1: Ask about Ethnicity • Question 2: Ask about Race WITH OPTION to select more than one racial designation 10

NIH Policy for Inclusion • OMB Directive 15 Issued 1997 – Ethnic Categories: • Hispanic or Latino • Not Hispanic or Latino – Racial Categories: • American Indian or Alaska Native • Asian • Black or African American • Native Hawaiian or Other Pacific Islander • White 11

OMB Directive 15 1997 • Where can you find examples of data collection instruments that use the new OMB standards? – Many examples exist including: • PHS 398 Personal Data Form questions • 2000 US Census questions – Do not use the 5/01 Inclusion Enrollment Table or Target Table in PHS 398 to collect data from subjects 12

Update to NIH Inclusion Policy • NIH-Defined Phase III Clinical Trials – Evidence must be reviewed to show whether clinically important sex/gender and race/ethnicity differences in intervention effect are expected – Plans for valid analysis must be included in the design – Results of analyses must be reported to NIH 13

Requirements for NIH-Defined Phase III Clinical Trials • Research plan must include one of the following: – Prior studies support significant differences between subgroups: – Need plans to conduct valid analyses to detect significant differences between sex/gender and/or racial/ethnic subgroups • For the purpose of this policy, Significant Difference is defined as a difference that is of clinical or public health importance based on substantial scientific data. This is not the same as “statistically significant difference. ” 14

Requirements for NIHDefined Phase III Clinical Trial Applications OR: – Prior studies support no significant differences between subgroups: • Representation as subject selection criterion is not required; however, inclusion and analyses are encouraged 15

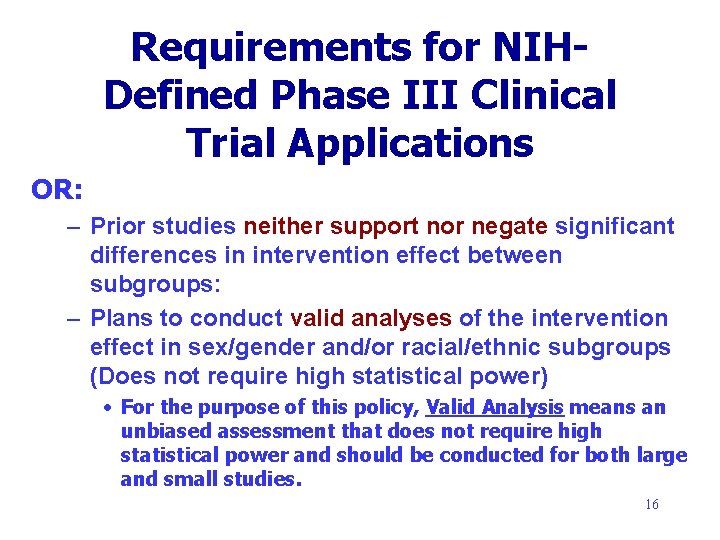
Requirements for NIHDefined Phase III Clinical Trial Applications OR: – Prior studies neither support nor negate significant differences in intervention effect between subgroups: – Plans to conduct valid analyses of the intervention effect in sex/gender and/or racial/ethnic subgroups (Does not require high statistical power) • For the purpose of this policy, Valid Analysis means an unbiased assessment that does not require high statistical power and should be conducted for both large and small studies. 16

Instructions in PHS 398 • Best source of information for investigators • http: //grants. nih. gov/ grants/funding/phs 398. html 17

Instructions in PHS 398 • Section E: Human Subjects Research üInclusion of Women üInclusion of Minorities • Failure to include = Return Application Prior to Review 18

Instructions in PHS 398 • Inclusion of Women and Minorities Sections must include: – Subject Selection Criteria & Rationale – Rationale for Any Exclusions – Enrollment dates (start and end) – Outreach Plans for Recruitment – Proposed Composition Using New Tables 19

Instructions in the 398 • NIH-Defined Phase III Clinical Trials – Evidence must be reviewed to show whether clinically important sex/gender and race/ethnicity differences in intervention effect are expected – Plans for valid analysis must be included in the design 20

Reviewer Instructions • Reviewers evaluate the Inclusion Plans http: //grants. nih. gov/grants/peer/hs_review_i nst. pdf • Unacceptable plans must be reflected in the priority score 21

Reviewer Instructions for Phase III Clinical Trials • Reviewers evaluate inclusion AND analysis plans http: //grants. nih. gov/grants/peer/hs_review_i nst. pdf • Unacceptable plans must be reflected in the priority score 22

Funding Decisions • Applications with Unacceptable Plans cannot be funded – must revise plans! 23

Monitoring Progress • Progress Reports must include Cumulative Enrollment – Reported using the Enrollment tables 24

Requirements for NIHDefined Phase III Clinical Trial Applications • Progress Reports need to include Both: – Enrollment Table – Statement in text about progress in data analyses for sex/gender and ethnicity/racial effects. 25

Additional Guidance on New Tables • NIH Policy on Reporting Race and Ethnicity Data: Subjects in Clinical Research • http: //grants. nih. gov/grants/guide/ notice-files/NOT-OD-01 -053. html 26

Additional Guidance on New Tables • New Application or Competing Continuation Involving Collection of New/Additional Data – Must use new 5/01 Table 27

Additional Guidance on New Tables • New Application or Competing Continuation with No Plans to Collect New/Additional Data – May use Either: • New 5/01 Table • 4/98 Version of the Inclusion Table – Use form appropriate for your data 28

Additional Guidance on New Tables • Non-Competing Applications (Progress Reports) for grants that began before FY 2002 – May use Either: • New 5/01 Table • 4/98 Version of the Inclusion Table – Use form appropriate for your data 29

30

Complying with the NIH Inclusion Policy • • Principal Investigators Review Staff and Reviewers Program Staff Grants Management Staff NIH Tracking and Inclusion Committee Congress Public 31

Monitoring Compliance with the NIH Inclusion Policy Annual Comprehensive Report: Monitoring Adherence to the NIH Policy on the Inclusion of Women and Minorities as Subjects in Clinical Research http: //www 4. od. nih. gov/orwh/inclusion. html 32

Resources and Getting Help • PHS 398 Instructions http: //grants. nih. gov/grants/funding/ph s 398/phs 398. html • PHS 2590 Instructions http: //grants. nih. gov/grants/funding/25 90/2590. htm 33

Resources and Getting Help • Inclusion of Women and Minorities – Implementation Page http: //grants. nih. gov/grants/fund ing/women_min. h tm • CONTACT IC PROGRAM STAFF! 34
 Problem statement example
Problem statement example What is the inclusion and exclusion criteria in research
What is the inclusion and exclusion criteria in research Inclusion criteria in research example
Inclusion criteria in research example Inclusion criteria in research example
Inclusion criteria in research example Nih roadmap for medical research
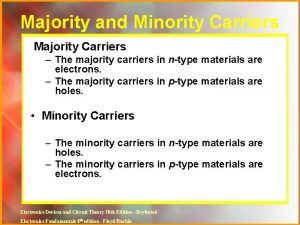
Nih roadmap for medical research Difference between majority and minority carriers
Difference between majority and minority carriers Indigenized dialect examples
Indigenized dialect examples Doctoral initiative on minority attrition and completion
Doctoral initiative on minority attrition and completion Society of clinical research associates
Society of clinical research associates Charlotte lemech
Charlotte lemech Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Good documentation practices in clinical research
Good documentation practices in clinical research Role of statistician in clinical trials
Role of statistician in clinical trials Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi institute of clinical research
Kavi institute of clinical research Academic research organizations
Academic research organizations Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research Asbmt clinical research training course
Asbmt clinical research training course Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Clinical research support services
Clinical research support services Clinical research definition
Clinical research definition Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Questra clinical research
Questra clinical research Mrc skills development fellowship
Mrc skills development fellowship Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Fsfv clinical trial
Fsfv clinical trial Example of inclusion and exclusion criteria
Example of inclusion and exclusion criteria California map to inclusion and belonging
California map to inclusion and belonging Diversity equity and inclusion 101
Diversity equity and inclusion 101 Diversity and inclusion scorecard
Diversity and inclusion scorecard