SEVERE COMBINED IMMUNE DEFICIENCY DISORDERS DIAGNOSIS AND THERAPEUTIC

SEVERE COMBINED IMMUNE DEFICIENCY DISORDERS: DIAGNOSIS AND THERAPEUTIC INTERVENTION IN PAST, PRESENT AND FUTURE TCT MEETING ORLANDO FEB 19 TH 2020 NEENA KAPOOR PROFESSOR OF PEDIATRICS CHILDREN’S HOSPITAL LOS ANGELES KECK SCHOOL OF MEDICINE USC

Disclosure Information Neena Kapoor, MD -No financial interest or conflict of interest

Outline of the talk • • • Introduction to SCID Presentation and diagnosis of SCID Supportive and curative treatment SCID, Hematopoietic stem cell transplant for SCID Gene therapy Future concerns and Issues 2
Primary Immune deficiency diseases (PID) (PID • Primary immune deficiency diseases( PIDD) comprise a heterogeneous group genetic of disorders that affects distinct components of the innate and adaptive immune system such as neutrophils, macrophages, dendritic cells, natural killer cells, T and B lymphocytes and complement components. • More than 300 distinct PID disorders have been identified. Spectrum of these diseases can vary from mild to sublethal to lethal disorders. • Lethality is due to uncontrolled infections, inflammation, autoimmunity and cancer. As most immune cells are derived from hematopoietic stem cells (HSC), HSCTs have long been considered a possible curative treatment of PIDDs. • First 200 patients transplanted between 1957 and 1967, including 12 patients with immunodeficiency disorders, none survived the procedure. HLA matching knowledge was in its infancy. Failure of procedure was mostly due to graft rejection and graft versus host disease. • In 1968, 2 patients with PIDD underwent successful transplant: one for severe combined immunodeficiency (SCID) and the other for Wiskott-Aldrich syndrome (WAS).
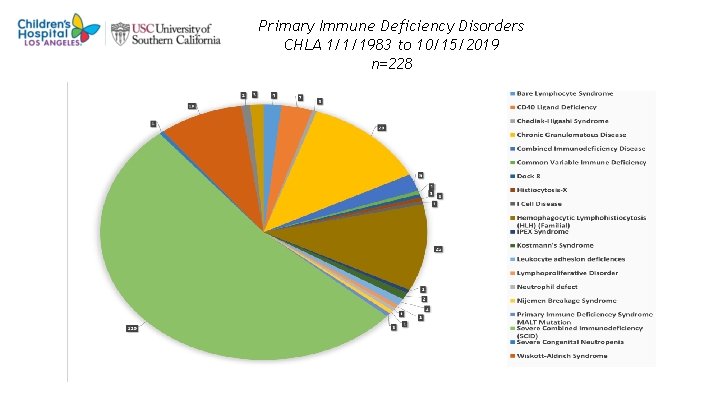
Primary Immune Deficiency Disorders CHLA 1/1/1983 to 10/15/2019 n=228 4

Severe combined immune deficiency (SCID): Bubble Boy Disease • SCID are hetrogenous group of genetic defects – In many, Mother carries the defective gene ( X linked) and disease expresses in male child, – Both parents may carry the gene for the disease ( autosomal recessive) and male or female child can be affected – Occasionally there may be spontaneous mutation in fetus and may cause the disease in male or female child • • Most of children with SCID do not produce T lymphocytes or they have non-functional T lymphocytes , or produce immunoglobulins or make specific antibodies Most of the children with SCID appear normal except for a few with dysmorphic features, congenital heart disease , skin and skeletal abnormalities. Most patients acquire multiple, persistent and severe viral, bacterial and fungal infections shortly after birth, fail to thrive, and rarely reach their first birthday with out definitive curative therapy. There are more than 35 different genetic defects have been identified that can cause SCID. Incidence of the disease may be 1: 40, 000 to 100, 000 live birth annual with 40 -100 new cases in USA each year Generally median age of diagnosis 4 -7 months of life In USA, with implementation of New born screening program (94% of all new born are screened with TREC assessment) diagnosis is within 1 st month of life. In California New born screening for SCID started in 2010. 5

SCID and Leaky SCID • • Classic SCID with unexplained lymphopenia ALC <500/ul. In the first few months of life any ALC <2, 500/μL is potentially pathogenic and may indicate SCID Infants with SCID who have T cells, showing slightly reduced, normal to high (T+SCID)or high T++SCID) T cell counts: ( Tricky SCID) – “nonfunctional” T+B+NK+SCID due to the defects of calcium channels flux – T+(CD 4+CD 8 -)B+NK+SCID in the defect of ZAP 70, – T++ SCID are most frequently due to abnormal and oligoclonal T cells very low naïve CD 4+CD 45 RA+T cells; high memory CD 4+CD 45 RO+T cells; high activated CD 3 DR+T cells; very low/absent TRECs, – SCID with engraftment of trans-placentally derived maternal T lymphocytes: • Omenn Syndrome : caused by hypomorphic mutations not only of RAG 1 -RAG 2 can be other genes whose null mutations cause instead typical SCID. – Have autologous oligoclonal hyper-autoreactive CD 4+TH 2 lymphocytes, – Lack of central and peripheral immunological tolerance – Thymic defect of thymocyte-dependent epithelial and dendritic cells and of AIRE (Autoimmune Regulator element) expression – Defect of CD 4+T Reg lymphocytes 6
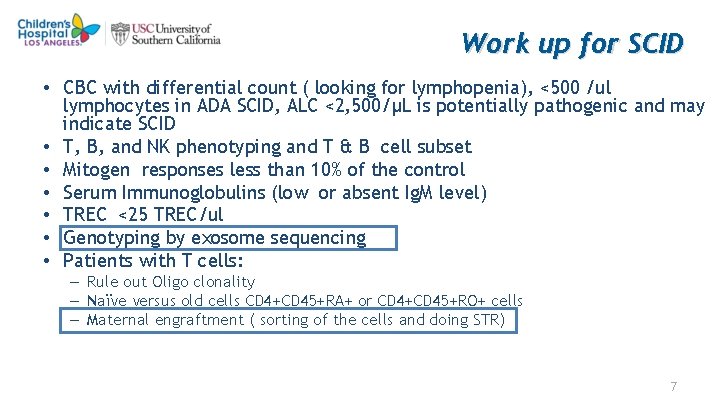
Work up for SCID • CBC with differential count ( looking for lymphopenia), <500 /ul lymphocytes in ADA SCID, ALC <2, 500/μL is potentially pathogenic and may indicate SCID • T, B, and NK phenotyping and T & B cell subset • Mitogen responses less than 10% of the control • Serum Immunoglobulins (low or absent Ig. M level) • TREC <25 TREC/ul • Genotyping by exosome sequencing • Patients with T cells: – Rule out Oligo clonality – Naïve versus old cells CD 4+CD 45+RA+ or CD 4+CD 45+RO+ cells – Maternal engraftment ( sorting of the cells and doing STR) 7

Classification of SCIDS • Defects in thymus embryogenesis • Impaired cytokine-mediated signaling • Pre-T cell receptor defects • Impaired signaling through the pre T cell receptor • Increased lymphocyte apoptosis • Other mechanisms • Impaired calcium flux 8
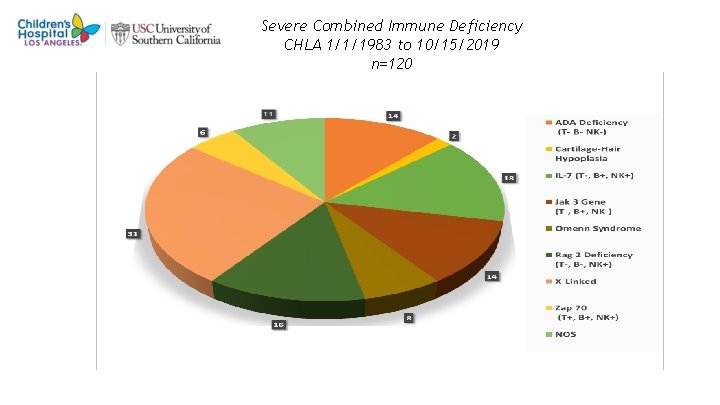
Severe Combined Immune Deficiency CHLA 1/1/1983 to 10/15/2019 n=120 9
Therapy • Hematopoietic stem cell transplant Potentially curative therapy for all types of SCID • Enzyme replacement therapy ADAGEN, Recombinant Adenosine Deaminase [PEG-r ADA], for ADA SCID • Gene therapy ADA SCID and X SCID • Protective isolation • Immunoglobulin replacement therapy • Prophylactic treatment for fungus, PJP, Viruses (These precautions must be observed till there is evidence of immunological reconstitution) 10

Last year celebrated 50 th Anniversary of this successful transplant • Since 1968 transplant from a Histocompatible sibling donor has been the gold standard in BMT
Essential Steps for Allogeneic Hematopoietic Stem Cell (HSC) Transplant • Identification of the patient with the disease treatable with HSC transplantation • Identification of suitable HSC donor • Conditioning regimen: No conditioning, Immunosuppression, Myeloablation and immunosuppression, Reduced intensity, Non-myeloablative • Infusion of the HSC • Post transplant immunosuppression • Recovery of lympho-hematopoiesis and immune reconstitution
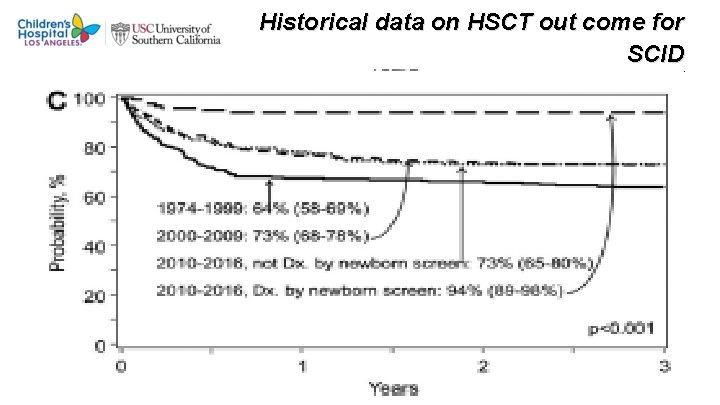
Historical data on HSCT out come for SCID R marsh et al. 2018 J ALLERGY CLIN IMMUNOLOGY 13
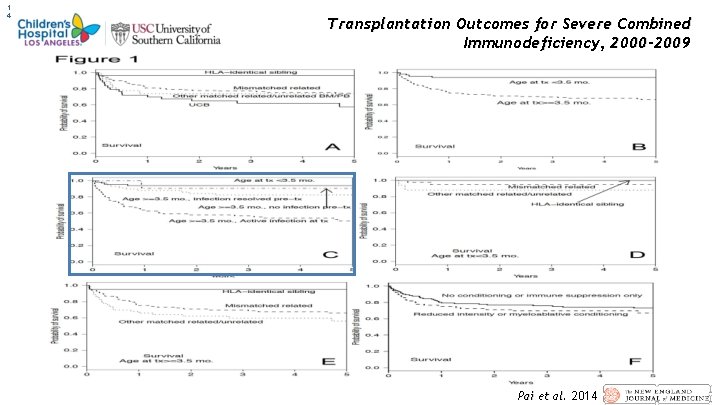
1 4 Transplantation Outcomes for Severe Combined Immunodeficiency, 2000– 2009 Pai et al. 2014
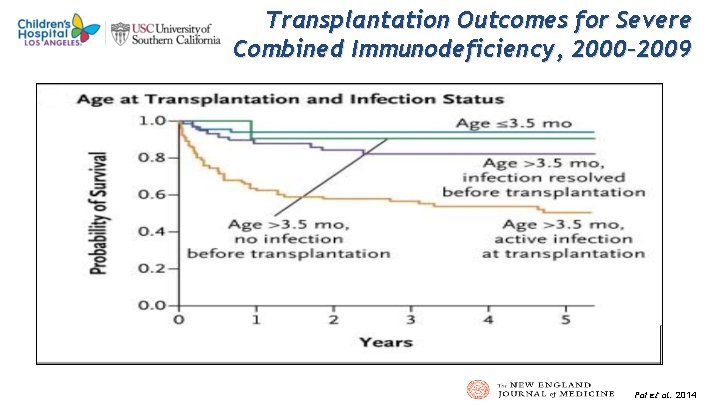
1 5 Transplantation Outcomes for Severe Combined Immunodeficiency, 2000– 2009 Pai et al. 2014
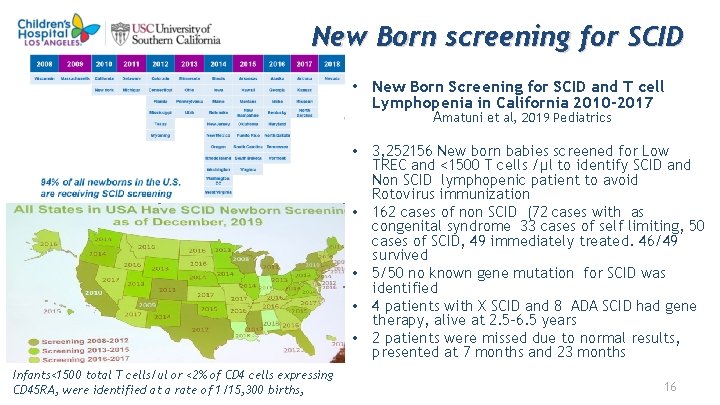
New Born screening for SCID • New Born Screening for SCID and T cell Lymphopenia in California 2010 -2017 Amatuni et al, 2019 Pediatrics • 3, 252156 New born babies screened for Low TREC and <1500 T cells /μl to identify SCID and Non SCID lymphopenic patient to avoid Rotovirus immunization • 162 cases of non SCID (72 cases with as congenital syndrome 33 cases of self limiting, 50 cases of SCID, 49 immediately treated. 46/49 survived • 5/50 no known gene mutation for SCID was identified • 4 patients with X SCID and 8 ADA SCID had gene therapy, alive at 2. 5 -6. 5 years • 2 patients were missed due to normal results, presented at 7 months and 23 months Infants<1500 total T cells/ul or <2% of CD 4 cells expressing CD 45 RA, were identified at a rate of 1/15, 300 births, 16
Infections and SCID • In a survey by Heimall et al Twenty-one of the 38 infants diagnosed via NBS experienced at least one infection prior to HCT (55%), compared to four of the 21 (19%) infants diagnosed via FH • These infections were cytomegalovirus (CMV) (n=5), Epstein-Barr virus (EBV) (n=2), and other respiratory viral infections (n=9) • All 5 infants with CMV infections were diagnosed via NBS (4 inpatient and 1 initially outpatient), none in those diagnosed via FH (p=0. 150). • Two year post-HCT survival was comparable in the subgroups: NBS (89%) vs FH (89%) (p=0. 897). 17

Treatment for SCID Hematopoietic stem cell transplant: suitable Donor options • Since 1968 BMT from a histo-compatible sibling donor is the gold standard • For a patient with genetic disease there is <25% chance to have histo-compatible sibling who is also healthy • Less than 5% have other related matched donor • 60 to 75% must have alternative Stem cell donor Unrelated marrow or PBSC donor unrelated placental cord blood product – NMDP was established in 1987 Sponsored by DOD – There about 13 million volunteer donor in the registry and >850 K cord blood products. WMDA has 25 Million donors in the registry • • For 10 -44% of patients there are no matched sibling or unrelated donor or Cord Blood. Option for those Patients : Haploidentical donor transplants Issues: Histo-incompatible post Thymic T Cells can lead to lethal graft versus host disease. Solution: Transplant can be undertaken following in Vivo or in Vitro T cell depletion 18
Haploidentical transplants Techniques used for depletion of T cells • T depletion with Soy Bean Lectin agglutination and E rosette • OKT 3 monoclonal antibodies depletion. • T cells depletion with use of Campath in the Bag • CD 34+ cell selection • Post graft Cyclophosphamide (In vivo depletion) • TCR αβ and CD 19 depletion ( in vitro T cell depletion) • CD 45 RA+ cell depletion (in vitro naïve T cell depletion) 19

2 0 Recipient of 1 st successful haplo-matched donor transplantation In Oct 1980 Marrow was T cell depleted with Soy Bean Lectin and E rosetting Technique Lesson learnt: Ø Graft failure, problem was overcome by giving mega doses of stem cells Ø T cell engraftment was achieved but not B cell engraftment with out myeloablative (SCID transplants were with out myeloablation) Ø Delayed immunological recovery 9 -12 month Ø In some cases due to inadequate depletion Graft versus Host disease Reisner Y, Kapoor N, et al Lancet 1981
2 1 David Vetter, famous Bubble Boy, received haploidentical marrow graft following depletion of T cells with Monoclonal anti OKT 3 antibody in 1982 Lesson learnt: This procedure selectively depleted T cell leaving behind B cells in the graft. This patient developed EBV induced lympho-proliferative disease in donor derived B cells. Shearer WT, Ritz J, et al N Engl J Med 1985
Haploidentical transplants • Techniques used for depletion of T cells • T depletion with Soy Bean Lectin agglutination and E rosette • OKT 3 monoclonal antibodies depletion. • T cells depletion with use of Campath in the Bag • CD 34+ cell selection • Post graft Cyclophosphamide (In vivo depletion) • TCR αβ and CD 19 depletion ( in vitro T cell depletion) • CD 45 RA+ cell depletion (in vitro naïve T cell depletion) 22
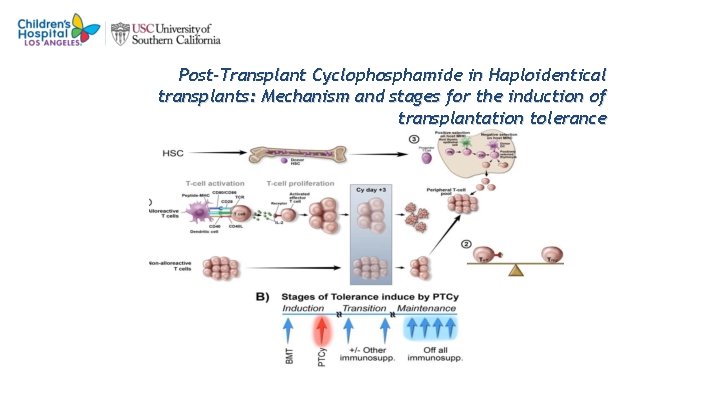
2 3 Post-Transplant Cyclophosphamide in Haploidentical transplants: Mechanism and stages for the induction of transplantation tolerance
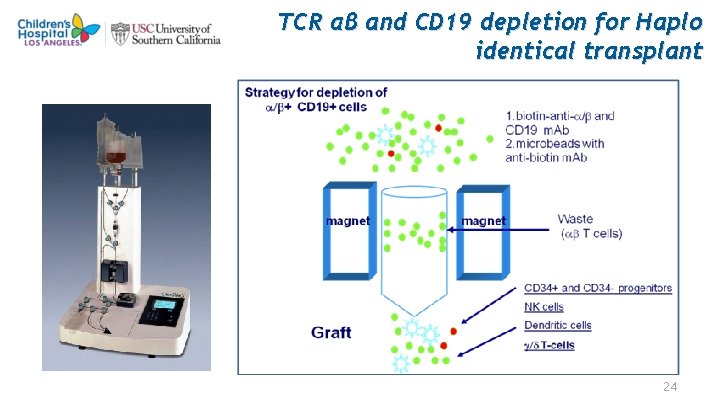
TCR αβ and CD 19 depletion for Haplo identical transplant 24
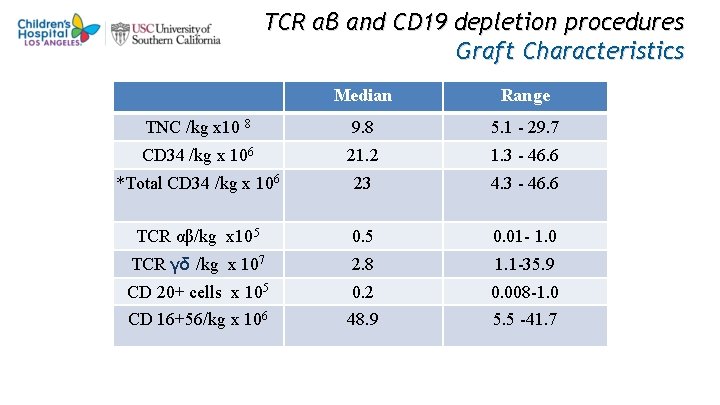
TCR αβ and CD 19 depletion procedures Graft Characteristics Median Range TNC /kg x 10 8 9. 8 5. 1 - 29. 7 CD 34 /kg x 106 21. 2 1. 3 - 46. 6 *Total CD 34 /kg x 106 23 4. 3 - 46. 6 TCR αβ/kg x 105 0. 01 - 1. 0 TCR γδ /kg x 107 2. 8 1. 1 -35. 9 CD 20+ cells x 105 0. 2 0. 008 -1. 0 CD 16+56/kg x 106 48. 9 5. 5 -41. 7
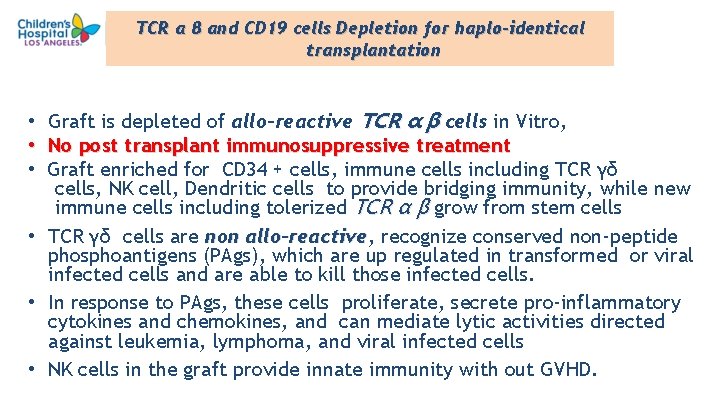
TCR α β and CD 19 cells Depletion for haplo-identical transplantation Graft is depleted of allo-reactive TCR α β cells in Vitro, No post transplant immunosuppressive treatment Graft enriched for CD 34 + cells, immune cells including TCR γδ cells, NK cell, Dendritic cells to provide bridging immunity, while new immune cells including tolerized TCR α β grow from stem cells • TCR γδ cells are non allo-reactive, allo-reactive recognize conserved non-peptide phosphoantigens (PAgs), which are up regulated in transformed or viral infected cells and are able to kill those infected cells. • In response to PAgs, these cells proliferate, secrete pro-inflammatory cytokines and chemokines, and can mediate lytic activities directed against leukemia, lymphoma, and viral infected cells • NK cells in the graft provide innate immunity with out GVHD. • • • 26
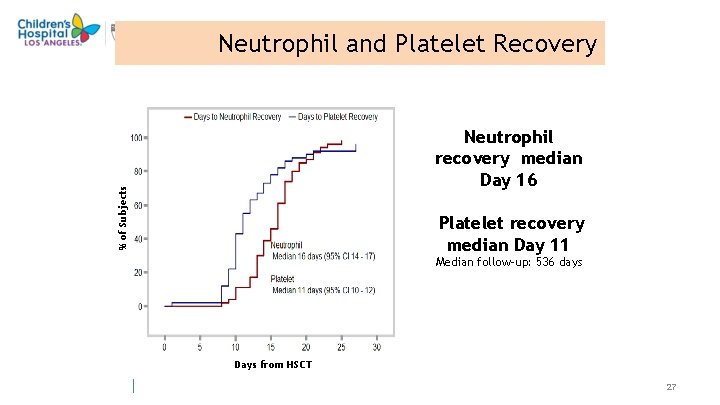
Neutrophil and Platelet Recovery % of Subjects Neutrophil recovery median Day 16 Platelet recovery median Day 11 Median follow-up: 536 days Days from HSCT 27
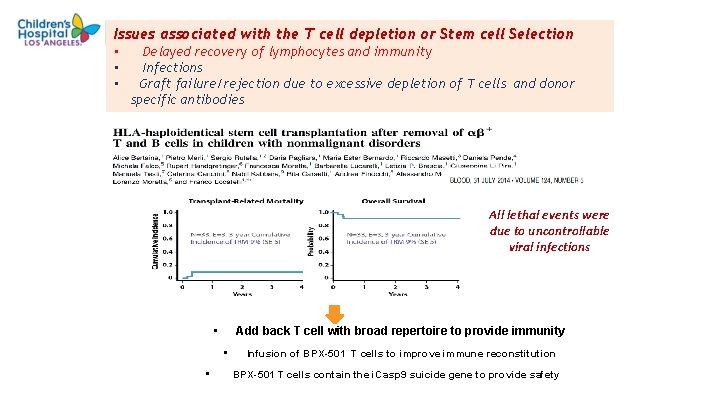
Issues associated with the T cell depletion or Stem cell Selection • • • Delayed recovery of lymphocytes and immunity Infections Graft failure/rejection due to excessive depletion of T cells and donor specific antibodies All lethal events were due to uncontrollable viral infections • Add back T cell with broad repertoire to provide immunity • • Infusion of BPX-501 T cells to improve immune reconstitution BPX-501 T cells contain the i. Casp 9 suicide gene to provide safety
Haploidentical transplants • Issues associated with the procedures • • • Stem cell loss during T cell depletion procedure Graft failure Graft versus Host disease Delayed recovery of lymphocytes and functional immunity Infections and re-occurrence of disease PTLD 29
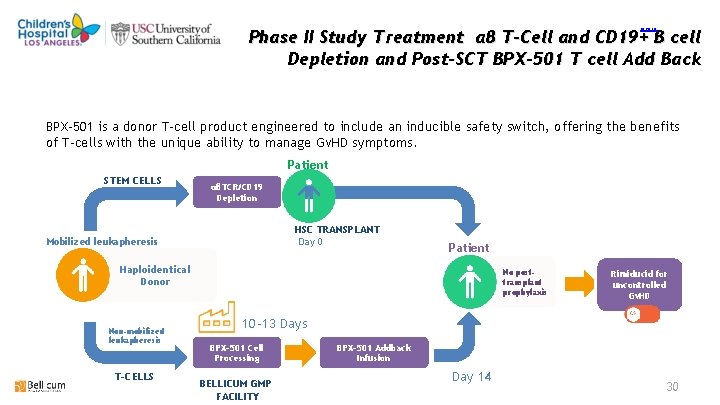
return Phase II Study Treatment αβ T-Cell and CD 19+ B cell Depletion and Post-SCT BPX-501 T cell Add Back BPX-501 is a donor T-cell product engineered to include an inducible safety switch, offering the benefits of T-cells with the unique ability to manage Gv. HD symptoms. Patient STEM CELLS αβTCR/CD 19 Depletion HSC TRANSPLANT Day 0 Mobilized leukapheresis Patient Haploidentical Donor Non-mobilized leukapheresis T-CELLS No posttransplant prophylaxis Rimiducid for uncontrolled Gv. HD 10 -13 Days BPX-501 Cell Processing BELLICUM GMP FACILITY BPX-501 Addback Infusion Day 14 30
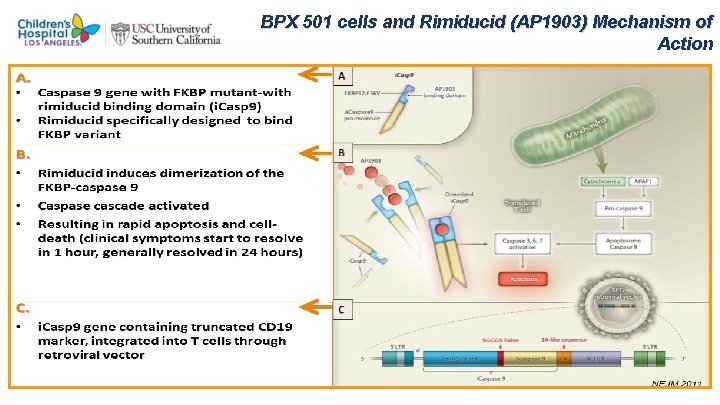
BPX 501 cells and Rimiducid (AP 1903) Mechanism of Action
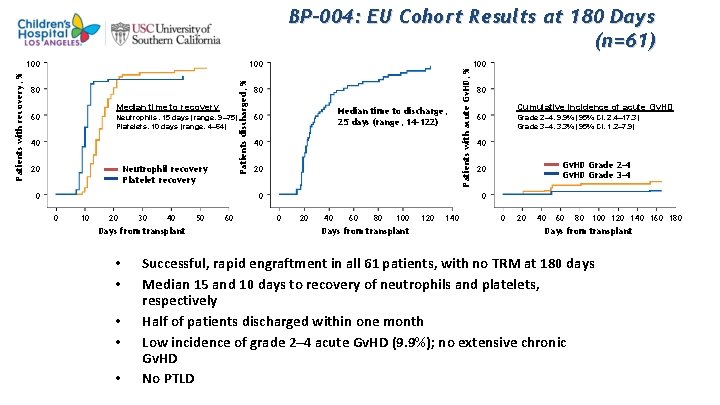
100 Patients discharged, % Patients with recovery, % 100 80 Median time to recovery 60 Neutrophils, 15 days (range, 9– 75) Platelets, 10 days (range, 4– 64) 40 20 Neutrophil recovery Platelet recovery 0 80 Median time to discharge, 25 days (range, 14– 122) 60 40 20 0 0 10 20 30 40 Days from transplant • • • 50 60 Patients with acute Gv. HD, % BP-004: EU Cohort Results at 180 Days (n=61) 100 80 Cumulative incidence of acute Gv. HD 60 Grade 2– 4, 9. 9% (95% CI, 2. 4– 17. 3) Grade 3– 4, 3. 3% (95% CI, 1. 2– 7. 9) 40 Gv. HD Grade 2– 4 Gv. HD Grade 3– 4 20 0 0 20 40 60 80 100 Days from transplant 120 140 0 20 40 60 80 100 120 140 160 180 Days from transplant Successful, rapid engraftment in all 61 patients, with no TRM at 180 days Median 15 and 10 days to recovery of neutrophils and platelets, respectively Half of patients discharged within one month Low incidence of grade 2– 4 acute Gv. HD (9. 9%); no extensive chronic Gv. HD No PTLD
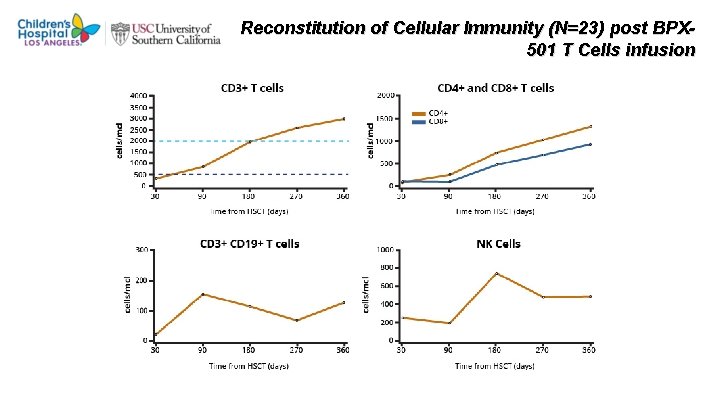
Reconstitution of Cellular Immunity (N=23) post BPX 501 T Cells infusion
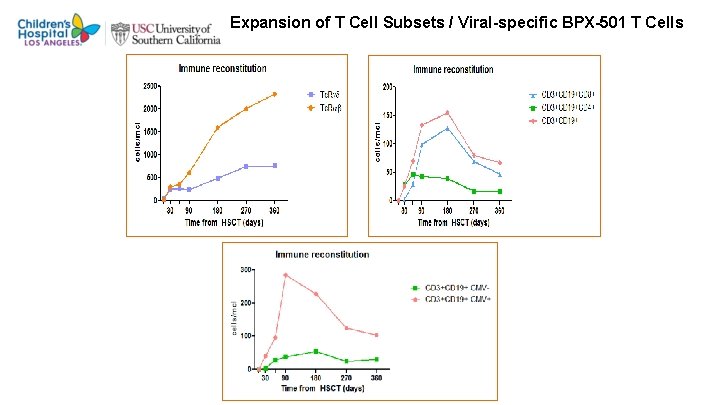
Expansion of T Cell Subsets / Viral-specific BPX-501 T Cells
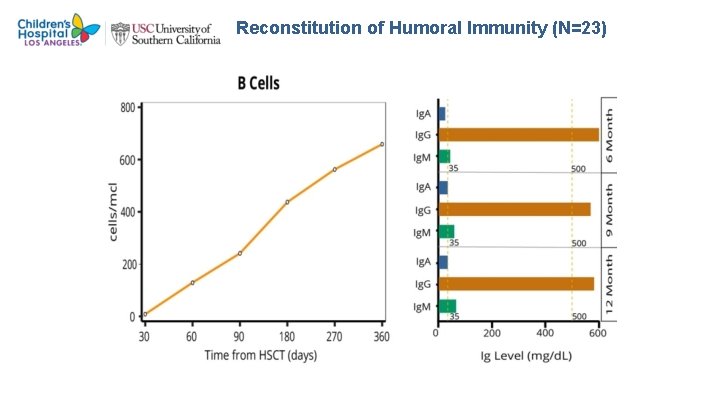
Reconstitution of Humoral Immunity (N=23)
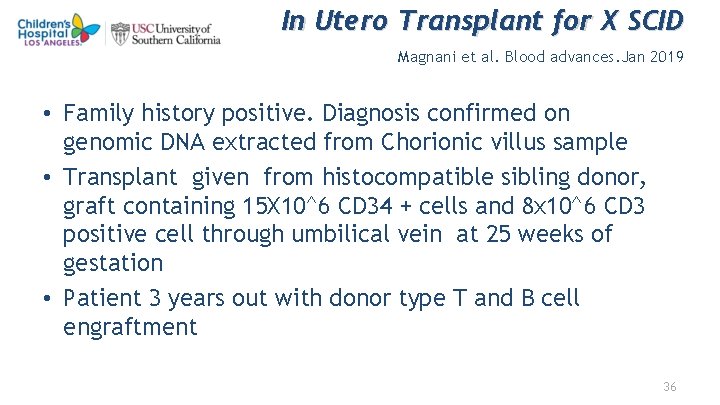
In Utero Transplant for X SCID Magnani et al. Blood advances. Jan 2019 • Family history positive. Diagnosis confirmed on genomic DNA extracted from Chorionic villus sample • Transplant given from histocompatible sibling donor, graft containing 15 X 10^6 CD 34 + cells and 8 x 10^6 CD 3 positive cell through umbilical vein at 25 weeks of gestation • Patient 3 years out with donor type T and B cell engraftment 36
Treatment of X-Linked Severe Combined Immunodeficiency by in Utero Transplantation of Paternal Bone Marrow: Flake…. Zanjani et al N Engl J Med 1996 • Diagnosis of SCID confirmed by DNA analysis at 12 weeks of gestation by chrionic villus sampling • Fetus was given three CD 34 enriched graft from Father Cell dose 14. 8 X 10^6, 2 X 10^6 and 1. 8 X 10^6 cells by ultra sound guided intra peritoneal graft. • 11 months follow up of the report patient remain immunologically reconstituted. 37

Questions which had been debated for years • CONDITION OR NOT TO CONDITION PRIOR TO TRANSPLANT FOR IMMUNE DEFICIENCY DISORDERS IF CONDITIONED, HOW THEY SHOULD BE CONDITIONED MYELOABLATIVE THERAPY, REDUCED TOXICITY, NONMYELOABLATIVE 38

Conditioning Regimens for SCID transplant Standard Conditioning regimen • Historically initially no conditioning was used to transplant SCID patient • In 80’s immunosuppression to prepare recipient to accept the graft: serotherapy ATG and or Cytoxan • In 90’s to achieve T and B cell engraftment myeloablative with Busulfan, Cytoxan and sero therapy was a common practice • In 2000 onwards Busulfan or Treosulfan with fludarabine and ATG is common conditioning therapy was started and continues to be the trend now. • Conditioning appears to be more compelling not only for B cell engraftment but also long term persistence of T cells and generation of naive T cells
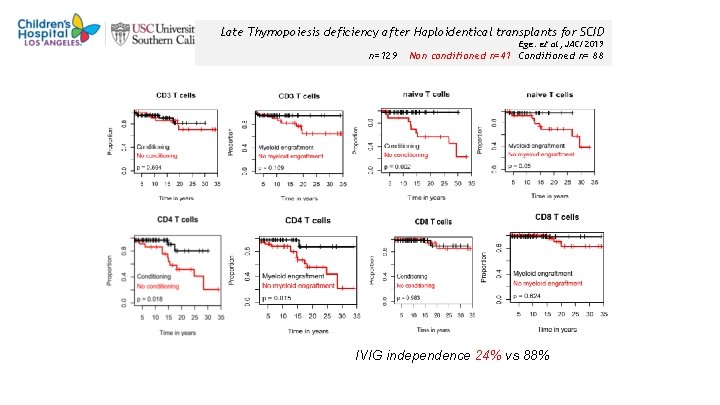
Late Thymopoiesis deficiency after Haploidentical transplants for SCID Ege. et al, JACI 2019 n=129 Non conditioned n=41 Conditioned n= 88 IVIG independence 24% vs 88% 40
Conditioning Regimens for SCID transplant • Issues related to conditioning regimen: • ---Predisposition to post transplant SOS and GVHD due to tissue injury -Other transplant conditioning related toxicity morbidity & mortality • Option: Reduced toxicity/ intensity Conditioning regimen • Modified regimen, use of same agents at lower dose with less toxicity, Bu, Flu, ATG regimen • Reduced intensity/non-myeloablative regimen just adequate to make transient space for the engraftment of donor derived all lineage of cells. PIDTC/ PBMTC sponsored study. • Concerns : • Mixed chimerism • ? Risk of autoimmune diseases • As observed before, will there be issues related to B cells and Stem Cells sustained engraftment 41
Phase I trial of Humanized monoclonal antibody AMG 191 (anti-CD 117 antibody) conditioning regimen for SCID Czechowicz Science 2007, Agarwal et al 2019 • Non Radiation or chemo therapy Conditioning regimen to deplete HSC and open up the niche for new stem cells engraftment. With humanized monoclonal antibody AMG 191 that binds to human CD 117 (c-Kit) to make space • 2/5 patients T- B- NK + SCID with DCLRE 1 C ( Artemis ) gene mutation , who previously had received unconditioned HSCT as infant, Had T cell engraftment, but no B cell engraftment received mobilized CD 34+ cells from original donor after AMG level was <100 ng/ml. • Beginning 8 weeks post transplant patients had CD 19+ CD 20 B cells of donor origin and also had Granulocytes of donor origin confirming stem cell engraftment. • Patients now are producing Immunoglobulins • Follow up is short 18 months. There is not complete ablation of marrow, Treatment just open up niches to engraft the cells ? How sustained these graft will be and will there be mixed chimerism 42

Gene Therapy for SCID • Primary immune deficiencies (PID) were amongst the first group of diseases to be effectively treated with gene therapy and this field has rapidly expanded over the past two decades with innovative new technologies being rapidly incorporated into clinical practice. • Correction of autologous hematopoietic stem cells (HSC) using viral vectors to deliver the appropriate transgene has changed the management landscape for these groups of patients. • The successful outcome of a number of clinical trials is bringing gene therapy towards a front line treatment option for selected conditions. • Younger, non infected patients have better outcome 43
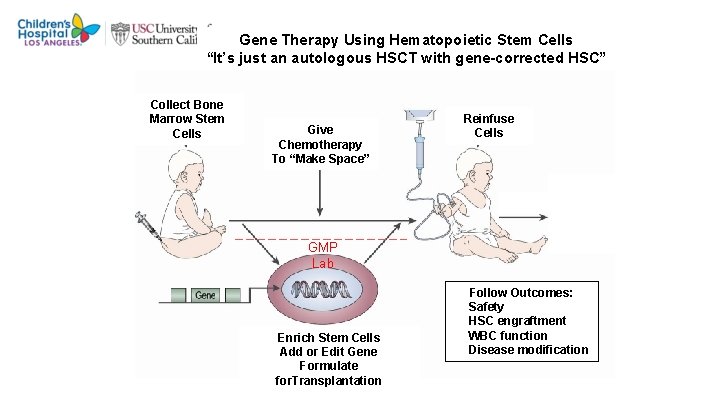
Gene Therapy Using Hematopoietic Stem Cells “It’s just an autologous HSCT with gene-corrected HSC” Collect Bone Marrow Stem Cells Give Chemotherapy To “Make Space” Reinfuse Cells GMP Lab Enrich Stem Cells Add or Edit Gene Formulate for. Transplantation Follow Outcomes: Safety HSC engraftment WBC function Disease modification
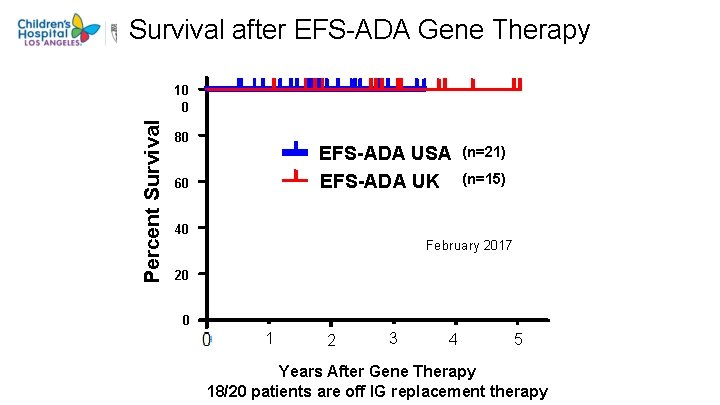
Survival after EFS-ADA Gene Therapy Percent Survival 10 0 80 EFS-ADA USA EFS-ADA UK 60 (n=21) (n=15) 40 February 2017 20 0 1 2 3 4 5 Years After Gene Therapy 18/20 patients are off IG replacement therapy
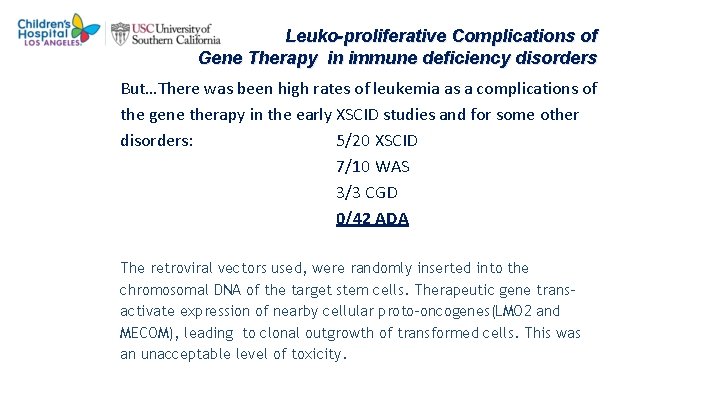
Leuko-proliferative Complications of Gene Therapy in immune deficiency disorders But…There was been high rates of leukemia as a complications of the gene therapy in the early XSCID studies and for some other disorders: 5/20 XSCID 7/10 WAS 3/3 CGD 0/42 ADA The retroviral vectors used, were randomly inserted into the chromosomal DNA of the target stem cells. Therapeutic gene transactivate expression of nearby cellular proto-oncogenes(LMO 2 and MECOM), leading to clonal outgrowth of transformed cells. This was an unacceptable level of toxicity.
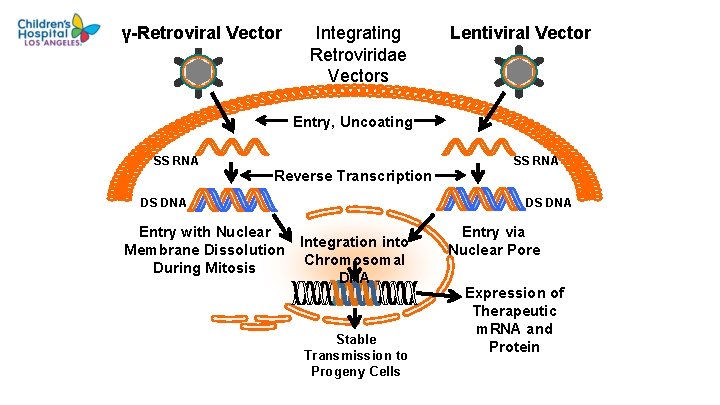
γ-Retroviral Vector Integrating Retroviridae Vectors Lentiviral Vector Entry, Uncoating SS RNA Reverse Transcription DS DNA Entry with Nuclear Membrane Dissolution During Mitosis SS RNA DS DNA Integration into Chromosomal DNA Stable Transmission to Progeny Cells Entry via Nuclear Pore Expression of Therapeutic m. RNA and Protein
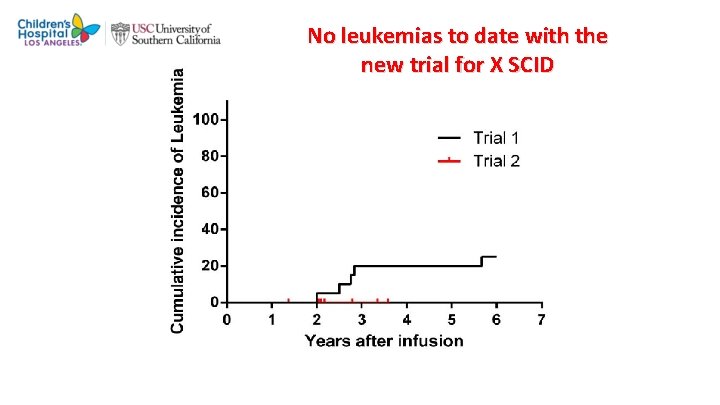
No leukemias to date with the new trial for X SCID Hacein-Bey-Abina, Pai et al, NEJM 2014

4 9 Gene therapy for X SCID • 20 infants with X-linked SCID have been treated with gene therapy. • Ninety five % exhibited engraftment of fully functional mature T cells, and most had sustained reconstitution. • Many also had sufficient humoral responses to eliminate the need for gamma globulin replacement, even though the fraction of transduced B cells was small and became undetectable six to ten years after therapy. • Normal transduced natural killer cells were also seen, in low numbers. Previously established infections were cleared, and no new serious infections have occurred in many of the patients who achieved reconstitution of T cell function. • Gene therapy was not successful in reconstituting immune function in four out of five older patients (age 10 to 20 years), several of whom had failed allogeneic HCT. • Subjective improvements in well-being were reported in two of the five patients
Gene-Editing Approaches for PID • Gene-editing technologies, including homology repair platforms based on…. – zinc-finger nucleases (ZFNs), – transcription activator-like effector nucleases (TALENs), – clustered regularly interspaced short palindromic repeats CRISPR/Cas 9. • The advantages over gene addition include: • Safety through precise editing of mutations. • Targeting specific gene loci to harness natural regulatory elements. • Targeting to “safe harbors” within the genome, which are known to tolerate transgene integration without risk for mutagenesis. 50
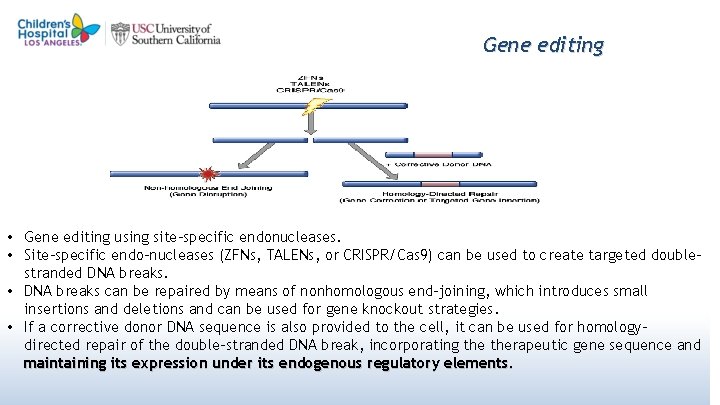
Gene editing • Gene editing using site-specific endonucleases. • Site-specific endo-nucleases (ZFNs, TALENs, or CRISPR/Cas 9) can be used to create targeted doublestranded DNA breaks. • DNA breaks can be repaired by means of nonhomologous end-joining, which introduces small insertions and deletions and can be used for gene knockout strategies. • If a corrective donor DNA sequence is also provided to the cell, it can be used for homologydirected repair of the double-stranded DNA break, incorporating therapeutic gene sequence and maintaining its expression under its endogenous regulatory elements. 51

In Summary • Severe combine immune deficiency disorders are heterogenous group of diseases curable with hematopoietic stem cell transplant • Evaluation of phenotyping and genotyping of the disease is important as that directs how these patient should be conditioned and transplanted. • Each one of these patients have been our teachers and have allowed us to understand the pathophysiology of the disease, taught us how best to intervene to correct the disease. • Concerns are long term effects of transplant procedures, including effect of oncogenic chemotherapy. Safer procedure are being investigated and are being developed. • Alternative treatment options like gene therapy and gene editing are coming up and are very promising • Longer follow up is necessary to determine the superiority of these newer procedures. 52

- Slides: 54