Seven Organic Reactions Combustion Substitution Addition Esterification Saponification

Seven Organic Reactions • Combustion • Substitution • Addition • Esterification • Saponification • Fermentation • Polymerization
Regents Chemistry Unit 7 - Organic Reactions Day 2 April 6, 2020
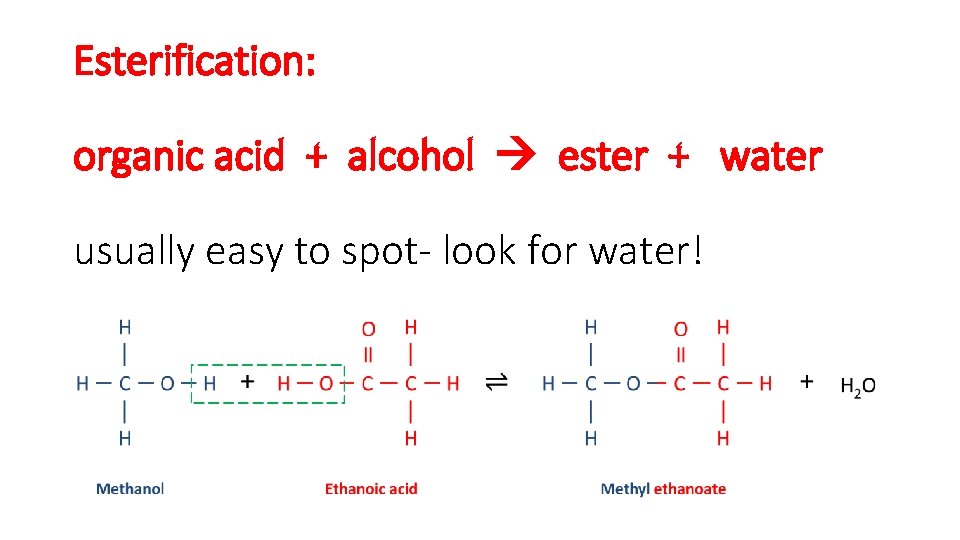
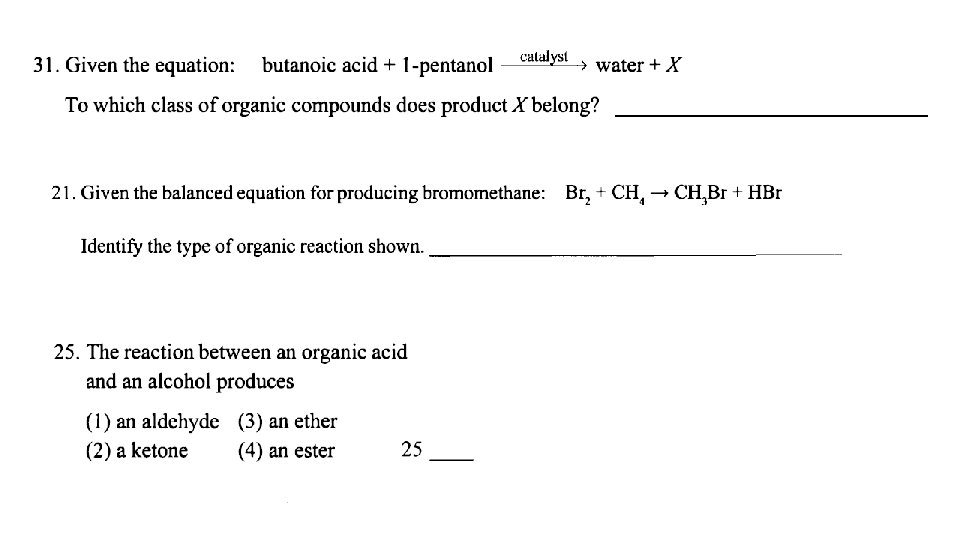
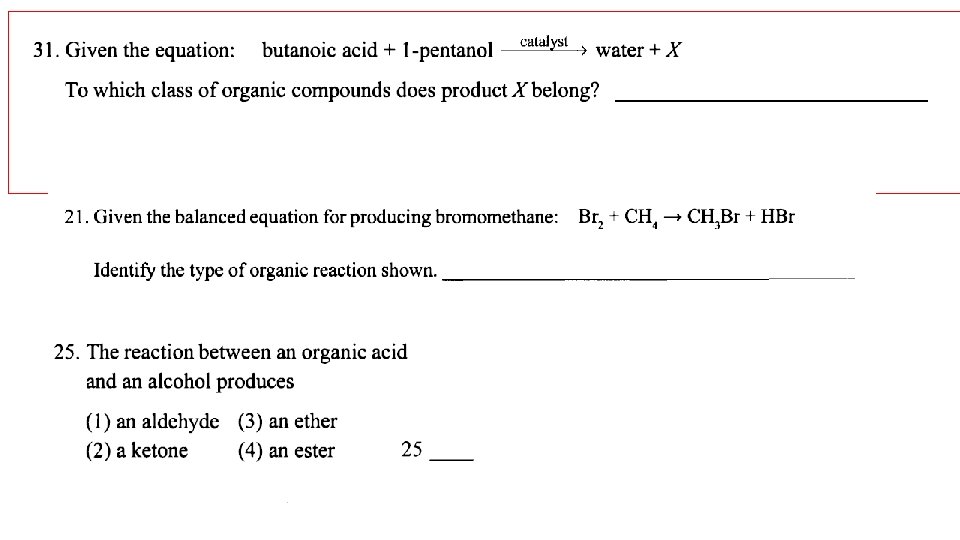
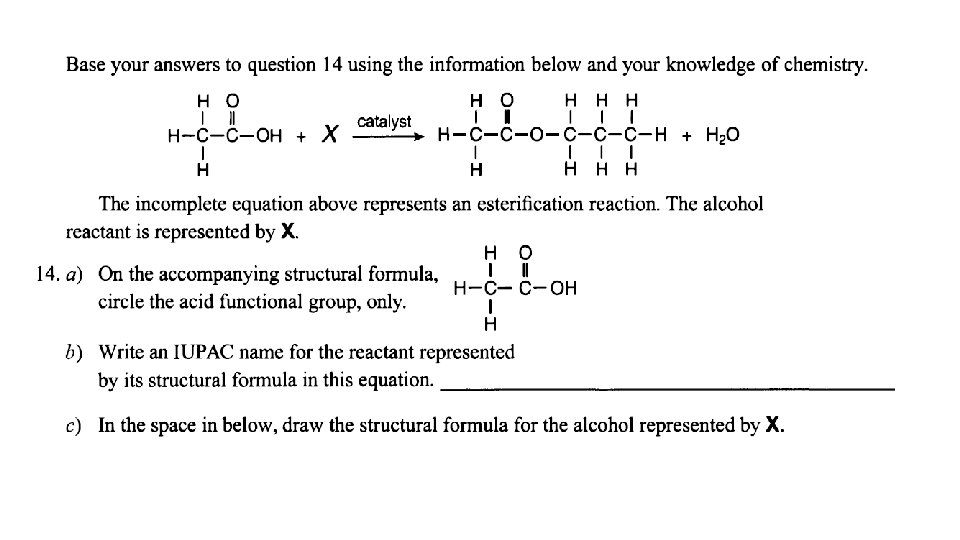
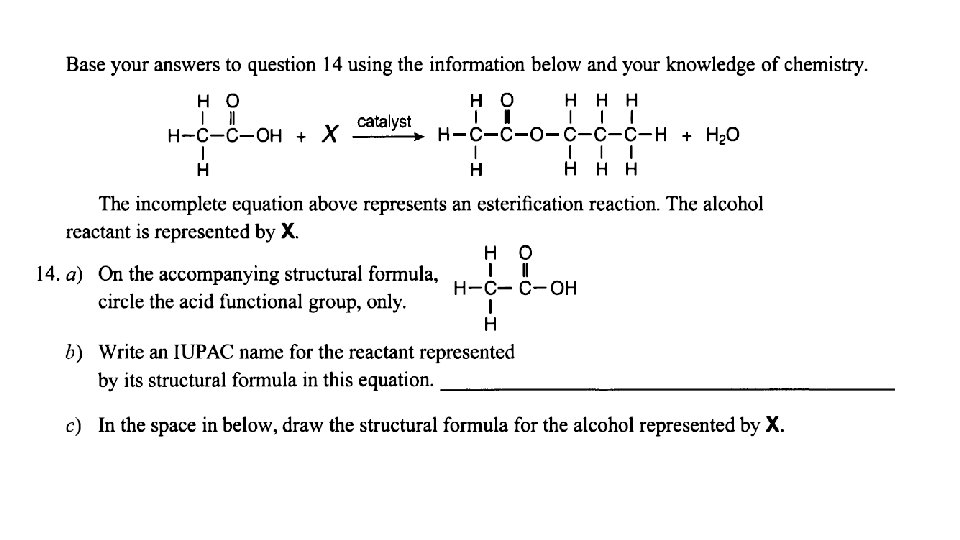
Esterification: organic acid + alcohol ester + water usually easy to spot- look for water!

Saponification: ester + base soap + alcohol
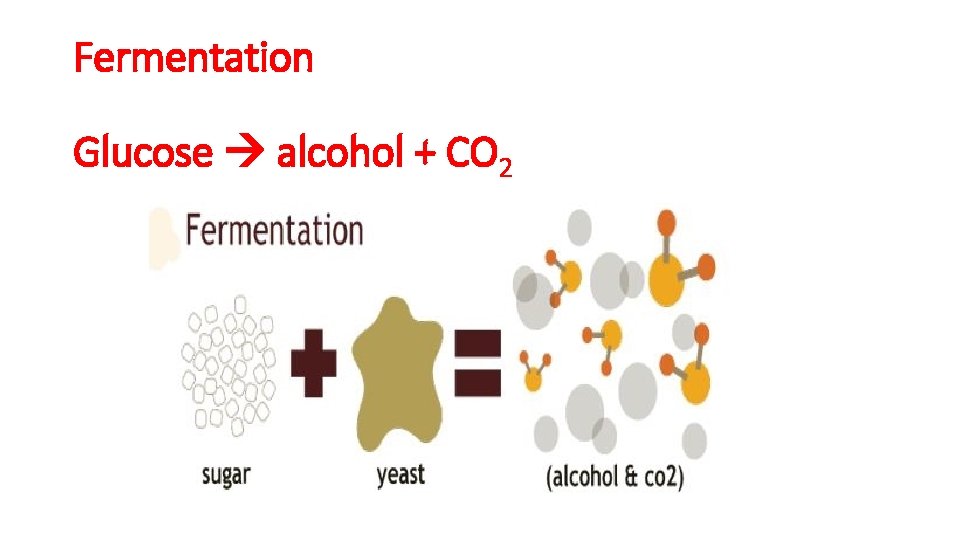
Fermentation Glucose alcohol + CO 2
Polymerization- formation of long chains of monomers covalently bonded as polymers • Two types: • Addition polymerization- joining small monomers of unsaturated hydrocarbons • Condensation polymerization- bonding monomers by removing water between them

Organic Reactions • Combustion- burning of hydrocarbon with oxygen to produce heat, CO 2, and H 2 O • Substitution- one atom or atoms is replaced by another atom or group of atoms • Addition- other atoms attach to carbons that are in a double or triple bond (removes multiple bond) • Esterification- organic acid+alcohol ester + water • Saponification- ester + base soap + alcohol • Fermentation- glucose breaks down into alcohol, CO 2, and energy • Polymerization- small organic units bond together to form very large molecules

Practice! Write the equation and name the reaction • 1 C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2 _____________ • 2 fat + base glycerol + soap _________ • 3 ethanoic acid + ethanol H 2 O + ethyl ethanoate • 4 C 2 H 4 + C 2 H 4 (C 2 H 4)n _____________________

Practice! Write the equation and name the reaction • 1 C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2 fermentation • 2 fat + base glycerol + soap saponification • 3 ethanoic acid + ethanol H 2 O + ethyl ethanoate • 4 C 2 H 4 + C 2 H 4 (C 2 H 4)n polymerization esterification
- Slides: 13