SESSION on Risk Characterization 1 Risk Characterization David












- Slides: 24

SESSION on Risk Characterization 1

Risk Characterization David Miller Chemist (USPHS) Health Effects Division Office of Pesticide Programs 2

Outline Summary of Preliminary Results/Findings Key Principles for Conducting a CRA § Hazard/Exposure Aspects Preliminary Cumulative Risk Assessment Time Frame Considerations Example Exposure Scenarios Questions to SAP 3

Summary of Preliminary Results/ Findings In general, consistent exposure/risk patterns across regions § Major contributor to risk is indoor residential uses of DDVP § Exposure through food is considered to be national and does not vary by region or season • performing additional analyses of these results § Drinking water and outdoor lawn and garden uses are not a significant contributor to risk 4

Key Principles for Conducting a Cumulative Risk Assessment Time Frame of Toxic Effect § What is the time to peak effect? § What is the time to recovery? Time Frame of Exposure § How often does exposure occur? § At what levels does exposure occur? § What is the exposure duration? How are Exposure and Toxicity compared? 5

Time-Frame Considerations September 2001 SAP Meeting considered this issue of how to compare time component of toxicity endpoints and exposure: § CRA should ideally compare toxicity endpoints and exposure durations of same time-frame § To the extent possible, comparison should take into account the pattern of human exposure 6

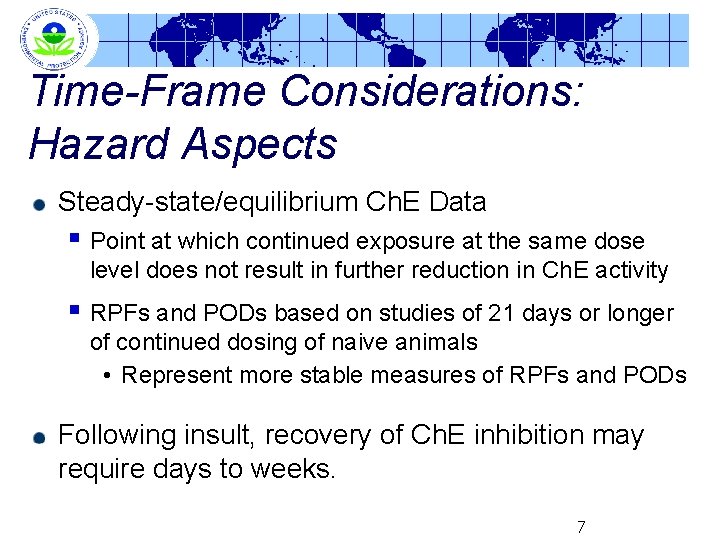
Time-Frame Considerations: Hazard Aspects Steady-state/equilibrium Ch. E Data § Point at which continued exposure at the same dose level does not result in further reduction in Ch. E activity § RPFs and PODs based on studies of 21 days or longer of continued dosing of naive animals • Represent more stable measures of RPFs and PODs Following insult, recovery of Ch. E inhibition may require days to weeks. 7

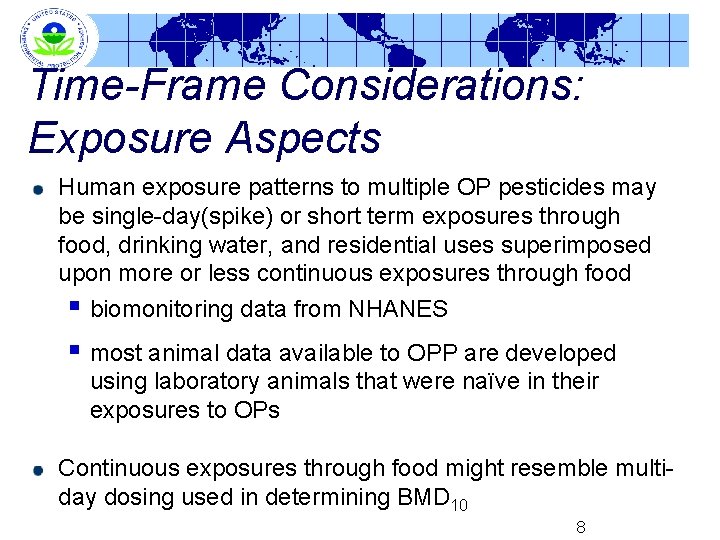
Time-Frame Considerations: Exposure Aspects Human exposure patterns to multiple OP pesticides may be single-day(spike) or short term exposures through food, drinking water, and residential uses superimposed upon more or less continuous exposures through food § biomonitoring data from NHANES § most animal data available to OPP are developed using laboratory animals that were naïve in their exposures to OPs Continuous exposures through food might resemble multiday dosing used in determining BMD 10 8

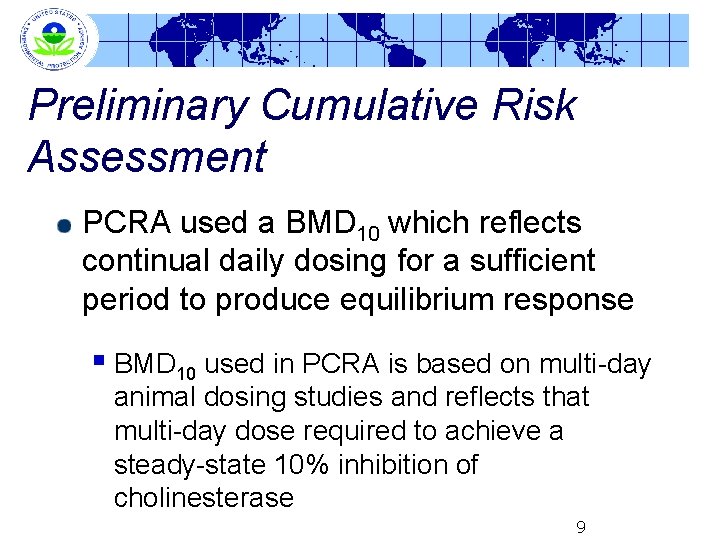
Preliminary Cumulative Risk Assessment PCRA used a BMD 10 which reflects continual daily dosing for a sufficient period to produce equilibrium response § BMD 10 used in PCRA is based on multi-day animal dosing studies and reflects that multi-day dose required to achieve a steady-state 10% inhibition of cholinesterase 9

Preliminary Cumulative Risk Assessment In the PCRA, OPP developed a distribution of single consecutive day exposures (NOT rolling time frame) and compared this to a steady-state (equilibrium) multi-day BMD 10 Consider “pattern” of exposures looking for sustained elevated exposures over a period of time § Recognize that such sustained elevated exposures at high percentiles are unlikely to reflect the same single individual 10

Time-Frame Considerations DEEM(FCID)/Calendex permits use of a “rolling time frame” approach § Exposures to the same individual are tracked over the time frame of interest and averaged for that individual over that time-frame. SAP considered this issue in previous session of this meeting 11

Time-Frame Considerations While this rolling time frame approach may allow for a better “match” between selected exposure time frames (e. g, 7 days or longer) and the hazard endpoint, OPP is concerned that this may not adequately permit estimates of risk associated with shorter duration exposures § e. g. , single day (spike) or short-term exposures 12

Time-Frame Considerations While an advantage of a rolling time frame approach is that it better simulates continual (non-naive) exposures and allows us to better match the time frame associated with the toxicological data, result of this “averaging” process may obscure oneday “spike” or elevated short-term exposures 13

Time-Frame Considerations A BMD 10 associated with a 21 day steadystate response is appropriate for 21 days or more If acute (1 -day) or short-term (<21 days) exposures are of concern, how might OPP evaluate/compare such exposures with toxicity data which is based on a multi-day BMD 10? § How does one estimate the effect of different exposure patterns on risk? 14

Time-Frame Considerations Some information is available with respect to how multi-day BMD 10 s compare to 1 -day NOAEL values § A rough comparison of BMD 10 s with NOAELs based on Ch. E data from single-dose studies reveals good similarity of values • Based on limited data set • Some exceptions 15

Example Exposure Scenarios NOTE: The example exposure scenarios will be provided on transparencies Discussion 16

Questions for the SAP on Risk Characterization 17

Question 1 There are several key principles for conducting a cumulative risk assessment. One such principle concerns the time frame of both the exposure (e. g. , What is the exposure duration? ) and of the toxic effect (e. g. , What are the time to peak effects and the time to recovery? ). Both must be adequately characterized prior to performing a cumulative risk assessment so that an individual’s exposure is matched with relevant toxicological values in terms of duration. There are several important considerations with respect to the temporal characteristics of the exposures and of the cholinesterase inhibitory effects of organophosphorus pesticides in estimating their cumulative risk. § There may be single day (spike) or short-term exposures to organophosphorus pesticides via food, nonoccupational/residential uses, and drinking water, as well as more or less continuous exposure via the diet (food). 18

Question 1 (continued) § In the Preliminary OP Cumulative Risk Assessment, OPP used relative potency factors and points of departure developed from cholinesterase inhibition in rats exposed to pesticides for 21 days or more. This practice was adopted to reflect cholinesterase inhibition at a point in the treatment schedule at which a steady state had been achieved. OPP elected to use data reflecting a steady state in the interest of producing relative potency factors (RPFs) that are reproducible and reflect less uncertainty due to rapidly changing time-sensitive measures of cholinesterase. In addition, when the compounds are at steady state, the differences in toxicokinetics among the OPs are less likely to impact the assessment. § OPP has information that indicates that the American population, in general, has some continuous level of exposure to OPs. Biomonitoring data from NHANES suggests that more than 80% of the American public have urinary metabolites indicating possible exposure to OPs. 19

Question 1 (continued) § § Most animal data available to OPP are developed using laboratory animals that were not previously exposed to OPs. In other words, the laboratory animals used in the toxicology studies were naive in their exposure to OPs. These studies show that OP’s can produce cholinesterase inhibition after a single exposure. A rough comparison of the BMD 10 s derived from female brain rat cholinesterase data from 21 days or longer duration with NOAELs based on cholinesterase data from single-dose studies reveals good similarity of values, with differences rarely exceeding two- to three-fold. Animal data suggest that recovery from a single exposure may take days to weeks. 20

Question 1 (continued) In light of all these factors, OPP wants to evaluate exposure across the most appropriate time frame(s). In the Preliminary OP Cumulative Risk Assessment, OPP developed a distribution of single consecutive day exposures, considering the pattern of MOEs occurring at a particular percentile of exposure across the calendar year. This approach focuses on exposure to the population of interest as a whole rather than attempting to track the variation in an individual's exposure from various sources of pesticide exposure. As an example, at the 95 th percentile of exposure, each day of the year will reflect a 95 th percentile exposure for the entire population and not reflect what may be lower, multi-day average exposures for any given individual. Calendex allows calculation of multi-day, rolling averages of exposure estimates for the individuals within the population. While this may allow for a match between selected exposure time frames (e. g. , 7 day or longer) and the hazard endpoint, OPP is concerned that this may not adequately permit estimates of risk associated with shorter duration exposures. 21

Question 1 (continued) Please comment on how best to evaluate risk, taking into account the temporal characteristics of the hazard endpoint (i. e. , cholinesterase inhibition) and the temporal characteristics of the exposure patterns for the food, drinking water, and residential/ nonoccupational pathways, with specific reference to: the pros and cons of various approaches of combining the exposure and hazard time frames to estimate cumulative risk, and methods to bound or estimate the biases in each approach. 22

Question 2 In the Preliminary OP Cumulative Risk Assessment, Section I. H lists a number of potential follow-up activities proposed by OPP. This list is not exhaustive. Does the Panel recommend any additional followup activities or sensitivity analyses beyond those listed? Does the Panel have any thoughts or recommendations about how these additional analyses should be conducted? Which activities should receive the greatest priority? 23

The End 24