Serotonin syndrome myth or reality Dr Yolande Knight




















- Slides: 27

Serotonin syndrome: myth or reality Dr Yolande Knight GP ST 2 BASH Gpw. SI meeting 15 March 2012

Serotonin syndrome Quick reminder about serotonin History What is it Clinical findings Diagnostic criteria Triptans Evidence

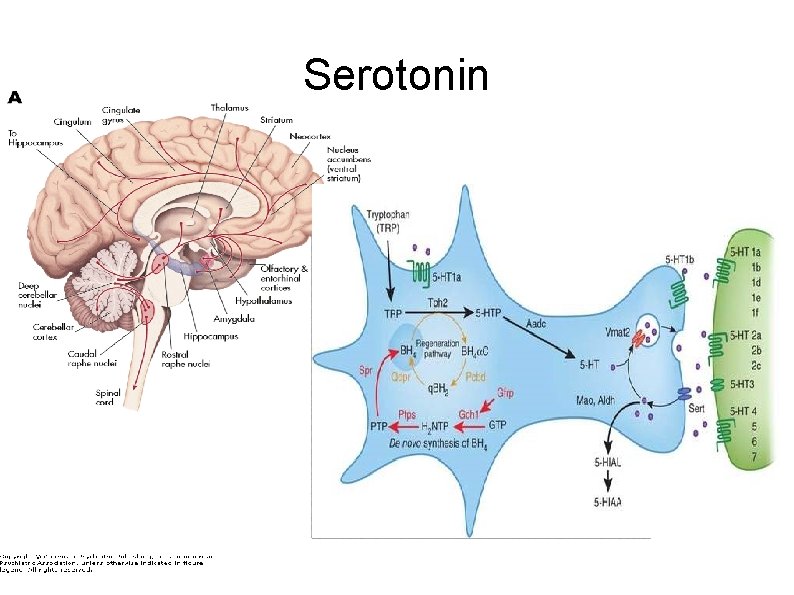
Serotonin or 5 -hydroxytryptamine (5 -HT) is a monoamine neurotransmitter Biochemically derived from trytophan Found in the GI tract, platelets and CNS 90% of the body's serotonin is in enterochromaffin cells in the gut, where it regulates intestinal movements Remainder is synthesised in CNS serotonergic neurons

Serotonin

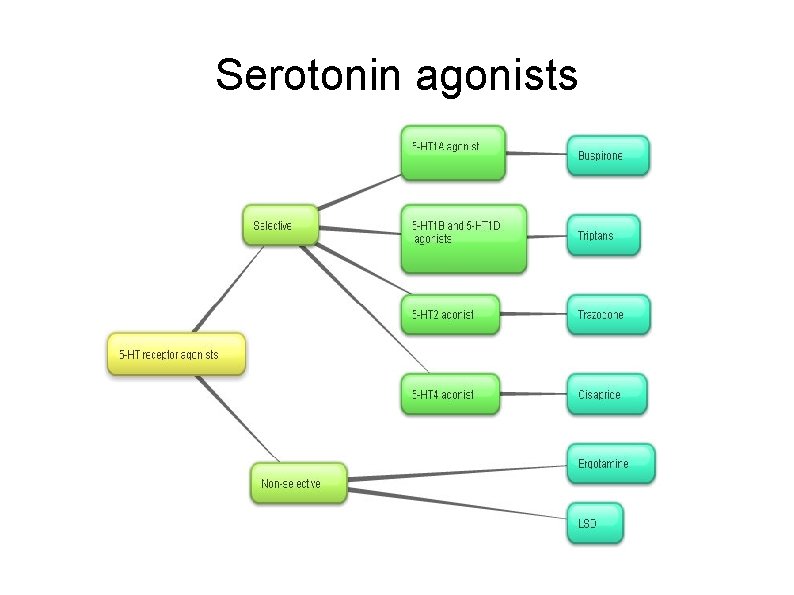
Serotonin agonists

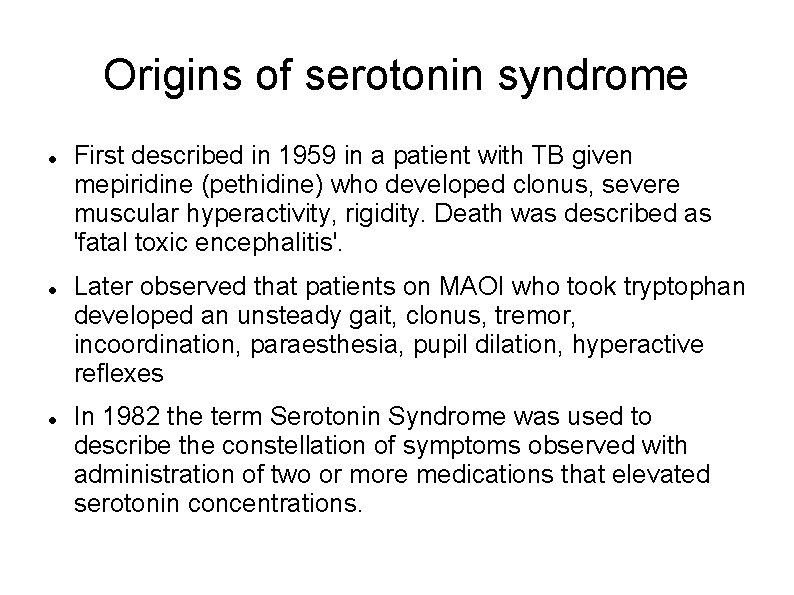
Origins of serotonin syndrome First described in 1959 in a patient with TB given mepiridine (pethidine) who developed clonus, severe muscular hyperactivity, rigidity. Death was described as 'fatal toxic encephalitis'. Later observed that patients on MAOI who took tryptophan developed an unsteady gait, clonus, tremor, incoordination, paraesthesia, pupil dilation, hyperactive reflexes In 1982 the term Serotonin Syndrome was used to describe the constellation of symptoms observed with administration of two or more medications that elevated serotonin concentrations.

Famous case of Libby Zion Libby Zion aged 18 y. o. died March 1984 in New York Hospital emergency dept. Had been taking phenelzine (MAOI) and cocaine. Junior doctors in ED gave mepiridine for 'jerking motions'. She developed agitation, T 42ºC then died of cardiac arrest. Later identified as serotonin syndrome. The treating doctors were sued for 38 counts of negligence for giving pethidine when they knew she took phenelzine, and for clinical negligence due to tiredness after working a 40 hour shift (father was a lawyer writing for the New York Times) Resulted in the Libby Zion Law 1989 restricting doctors to working 80 hours a week.

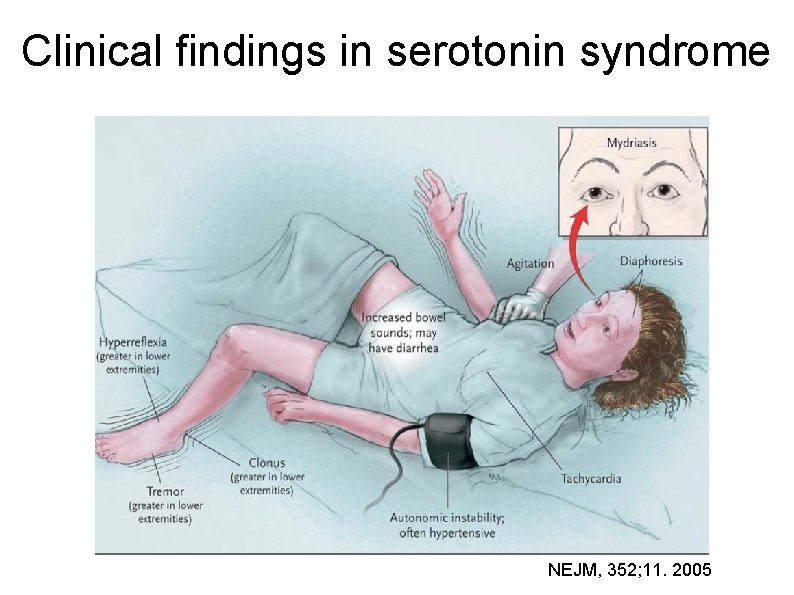
Serotonin syndrome (SS) or serotonin toxicity or serotonin-mediated morbidity, triad of altered mental status dysautonomia confusion, agitation, seizures diarrhoea, diaphoresis, hypertension, fever/shivering, mydriasis, tachycardia neuromuscular changes myoclonus, tremor, ataxia, hyperreflexia, rigidity

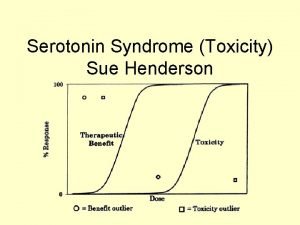
Spectrum of clinical findings NEJM, 352; 11. 2005

Clinical findings in serotonin syndrome NEJM, 352; 11. 2005

Diagnostic criteria- the Hunter criteria validated in >2000 SSRI overdoses

Pathophysiology Mediated via the final common pathway of elevated intra-synaptic serotonin Risk of serious morbidity arises from hyperthermia, which is mediated in a doseresponsive manner via 5 -HT 2 A receptors Reversed by 5 -HT 2 A antagonist Degree of 5 -HT elevation required for toxicity is 10 -50 times above baseline level

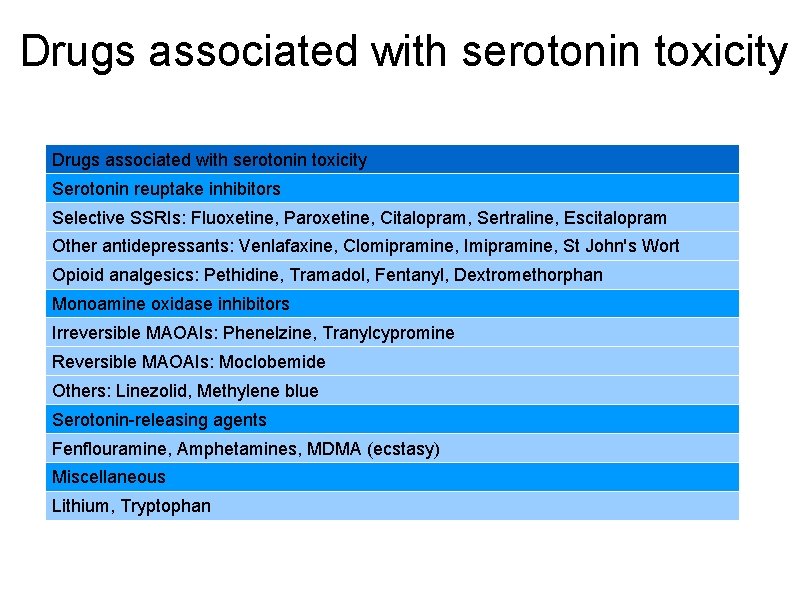
Drugs associated with serotonin toxicity Serotonin reuptake inhibitors Selective SSRIs: Fluoxetine, Paroxetine, Citalopram, Sertraline, Escitalopram Other antidepressants: Venlafaxine, Clomipramine, Imipramine, St John's Wort Opioid analgesics: Pethidine, Tramadol, Fentanyl, Dextromethorphan Monoamine oxidase inhibitors Irreversible MAOAIs: Phenelzine, Tranylcypromine Reversible MAOAIs: Moclobemide Others: Linezolid, Methylene blue Serotonin-releasing agents Fenflouramine, Amphetamines, MDMA (ecstasy) Miscellaneous Lithium, Tryptophan

Treatment Cyproheptadine and chlorpromazine are the 5 -HT 2 antagonists that have been used most extensively. Oral cyproheptadine (4– 12 mg) is probably the most useful 5 HT 2 antagonist for moderate toxicity. Limited usefulness in severe toxicity. In severe serotonin toxicity use IV chlorpromazine

Neurone NEJM, 352; 11. 2005

Examples of known drug combinations causing severe serotonin toxicity An irreversible MAOI (in normal dose) plus any serotonin reuptake inhibitor in normal dose – Tranylcypromine (or phenelzine) + clomipramine or venlafaxine or any SSRI – Tranylcypromine (or phenelzine) + tramadol or pethidine Any reverisble MAOAI in high dose (eg Moclobemide) + any SSRI in normal dose or SRI analgesic (e. g. tramadol) MDMA (ecstasy) + Moclobemide (but not SSRIs)

Epidemiology- serotonin toxicity In 2002 in US, 93 deaths and 7349 cases of significant toxicity due to SSRI exposure (Watson et al, 2003) Of SSRI overdoses serotonin syndrome occurs in 15% of cases (Isbister et al, 2004) 85% of GPs in a UK study were unaware of the diagnosis of serotonin syndrome (Mackay et al, BJGP 1999)

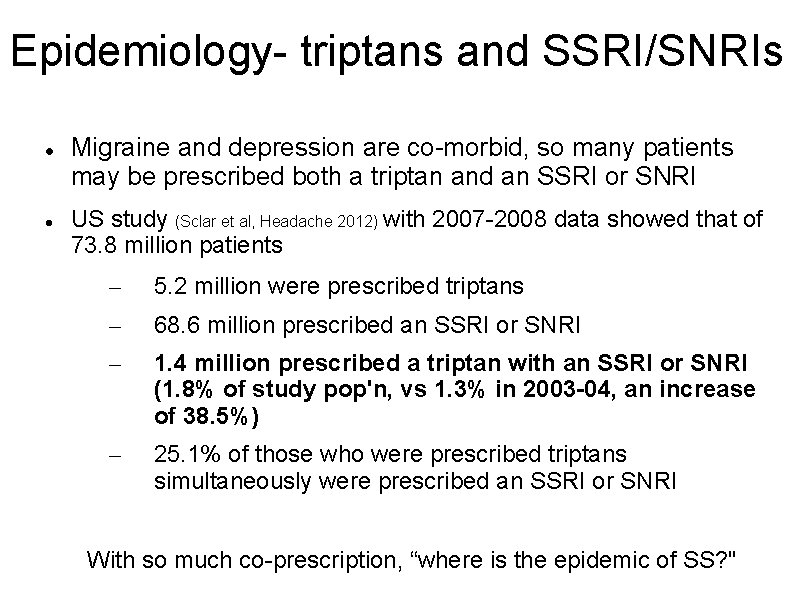
Epidemiology- triptans and SSRI/SNRIs Migraine and depression are co-morbid, so many patients may be prescribed both a triptan and an SSRI or SNRI US study (Sclar et al, Headache 2012) with 2007 -2008 data showed that of 73. 8 million patients – 5. 2 million were prescribed triptans – 68. 6 million prescribed an SSRI or SNRI – 1. 4 million prescribed a triptan with an SSRI or SNRI (1. 8% of study pop'n, vs 1. 3% in 2003 -04, an increase of 38. 5%) – 25. 1% of those who were prescribed triptans simultaneously were prescribed an SSRI or SNRI With so much co-prescription, “where is the epidemic of SS? "

Safety alert- warranted? In 2006 the US FDA (and supported by UK MHRA) issued a warning based on 27 'case reports' gathered over 5 years of triptan and SSRI/SNRI combination producing serotonin syndrome

Triptans- relevant pharmacology Mostly 5 -HT 1 B/1 D agonists (Kis ~10 n. M = potent) (excluding Sumatriptan which is purely 5 -HT 1 D, and Eletriptan which is 5 HT 1 B/1 D/1 F Very poor affinity at 5 -HT 2 A receptors (Kis ~10, 000 n. M = very weak) Safety data produced by drug companies on the triptans shows no signs of serotonin toxicity when overdose on triptan In rats given 100 times the usual dose of Naratriptan (30 mg/kg) no behavioural effects of SS were observed Other drugs with greater affinity for 5 -HT 1 A receptors also do not cause SS in overdose e. g. buspirone

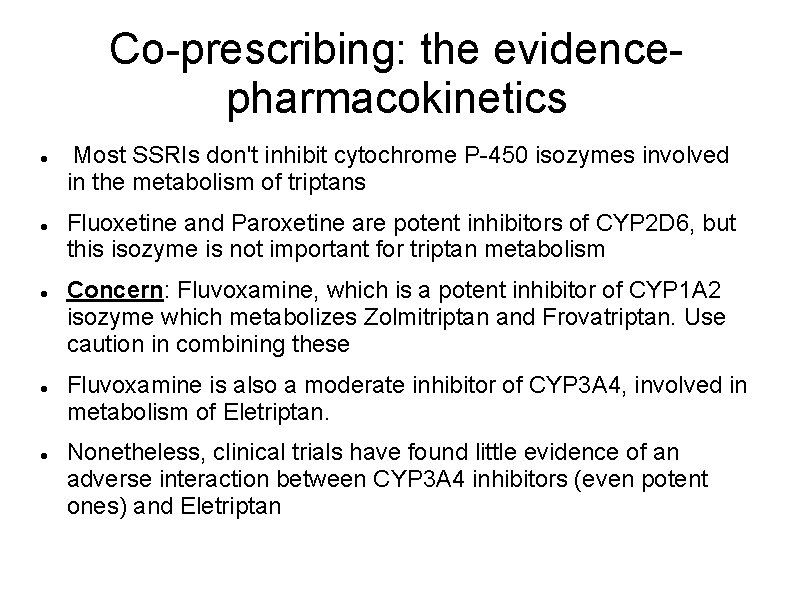
Co-prescribing: the evidencepharmacokinetics Most SSRIs don't inhibit cytochrome P-450 isozymes involved in the metabolism of triptans Fluoxetine and Paroxetine are potent inhibitors of CYP 2 D 6, but this isozyme is not important for triptan metabolism Concern: Fluvoxamine, which is a potent inhibitor of CYP 1 A 2 isozyme which metabolizes Zolmitriptan and Frovatriptan. Use caution in combining these Fluvoxamine is also a moderate inhibitor of CYP 3 A 4, involved in metabolism of Eletriptan. Nonetheless, clinical trials have found little evidence of an adverse interaction between CYP 3 A 4 inhibitors (even potent ones) and Eletriptan

Co-prescribing: the evidenceclinical data Dr. Randall Evans, using the Freedom of Information Act, (USA) obtained and reviewed the FDA cases Not one case met the Hunter criteria, and only 10 of the 29 met the Sternbach criteria. A number of the FDA case reports consisted of patient selfreports, and contained no symptoms suggestive of a diagnosis of SS.

The evidence- clinical data A second set of 11 patients was reported in the New England Journal of Medicine as proof of triptan monotherapy-induced serotonin syndrome (). The authors of this report did not describe whether either the Sternbach or Hunter criteria were met. The authors also suggested the symptoms remitted with supportive care or intravenous diphenhydramine, which is not a treatment for SS. Questionable diagnoses? 4 subsequent pro- and retrospective studies showed no clinically observable interaction between triptans and antidepressants (Blier et al J Clin Psychopharm 1995; Putnam et al Cephalalgia 1999, Gardner et al Ann Pharmacother 1998; Hettiarachi et al Cephalalgia 2001)

What explains the reports of serotonin syndrome in co-prescribed triptan and SSRI/SNRI? Some of the published case reports provided insufficient detail to determine if 1. SS was the diagnosis 2. the symptoms were caused by a drug acting alone, rather than drug interaction 3. the reaction was caused by some other drug given around the same time (within 5 weeks)

Are some patients at greater risk? Can't rule out that a triptan-SSRI/SNRI interaction could occur Possible that only certain patients develop SS when SSRI/SNRIs are used with triptans This could explain the rarity of case reports Genetic factors determine isozyme profile- differentially metabolise triptans and SSRI/SNRIs

Position Paper The American Headache Society (AHS) Position Paper on Serotonin Syndrome: – "With only Class IV evidence available in the literature and available through the FDA registration of adverse events, inadequate data are available to determine the risk of serotonin syndrome with the addition of a triptan to SSRIs/SNRIs or with triptan monotherapy. – “The currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U)”.

Myth or reality? Poor evidence base None of the FDA case reports use the diagnostic criteria, or report the unifying clinical feature for diagnosis (clonus) Proceed with caution?
 Ssri snri combination
Ssri snri combination Transcyclopromine
Transcyclopromine Zoos connect us to the natural world claim
Zoos connect us to the natural world claim Zoos myth and reality answers
Zoos myth and reality answers Yolande appelman
Yolande appelman Yolande pigaiani
Yolande pigaiani Yolande lucire
Yolande lucire Excitatory neurotransmitters function
Excitatory neurotransmitters function Serotonin vs dopamine
Serotonin vs dopamine Glycine serotonin
Glycine serotonin Serotonin
Serotonin Serotonin function
Serotonin function Serotonin definition
Serotonin definition Dopamine and serotonin pathways
Dopamine and serotonin pathways Serotonin obesity
Serotonin obesity Serotonin and chocolate
Serotonin and chocolate Blood features
Blood features Fleksiyon nedir
Fleksiyon nedir Castle vocabulary
Castle vocabulary La belle dame sans merci speaker
La belle dame sans merci speaker Knight frank prime central london
Knight frank prime central london N in japanese
N in japanese Leonardo da vinci ball bearing
Leonardo da vinci ball bearing The knight's tale middle english
The knight's tale middle english Bercilak de hautdesert
Bercilak de hautdesert Sir gawain fitt 3
Sir gawain fitt 3 The sea licked the grass at the edge of the shore.
The sea licked the grass at the edge of the shore. Sister knight
Sister knight