Sequential Parallel Comparison Design Elaine Hoffman Ph D






- Slides: 29

Sequential Parallel Comparison Design Elaine Hoffman, Ph. D, Statistical Science Director, PPD Anastasia Ivanova, Ph. D, UNC-CH, Dept. of Biostatistics


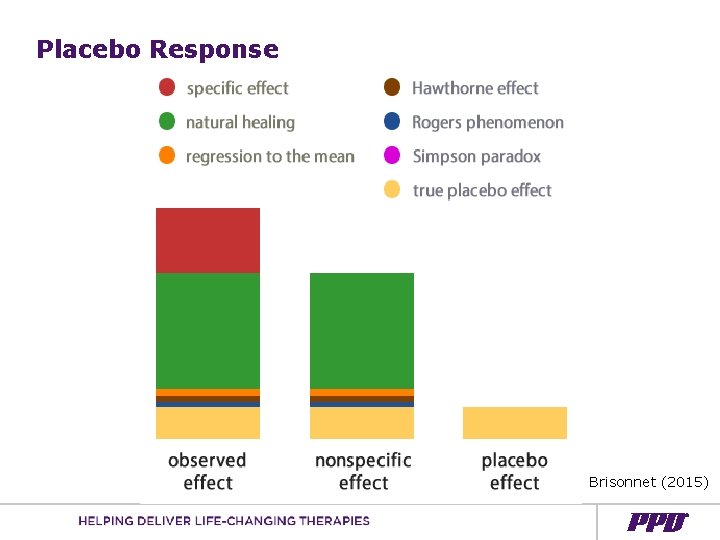
Placebo Response Brisonnet (2015)

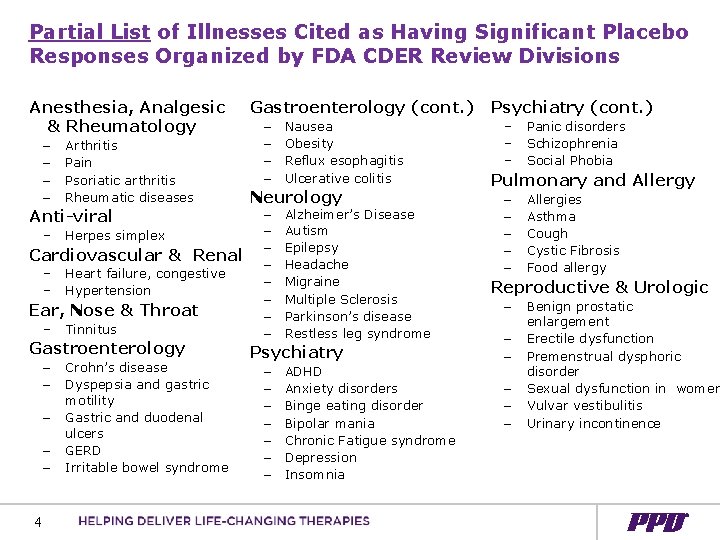
Partial List of Illnesses Cited as Having Significant Placebo Responses Organized by FDA CDER Review Divisions Anesthesia, Analgesic & Rheumatology – – Arthritis Pain Psoriatic arthritis Rheumatic diseases Anti-viral – Herpes simplex Cardiovascular & Renal – – Heart failure, congestive Hypertension Ear, Nose & Throat – Tinnitus Gastroenterology – – – 4 Crohn’s disease Dyspepsia and gastric motility Gastric and duodenal ulcers GERD Irritable bowel syndrome Gastroenterology (cont. ) – Nausea – Obesity – Reflux esophagitis – Ulcerative colitis Neurology – Alzheimer’s Disease – Autism – Epilepsy – Headache – Migraine – Multiple Sclerosis – Parkinson’s disease – Restless leg syndrome Psychiatry – ADHD – Anxiety disorders – Binge eating disorder – Bipolar mania – Chronic Fatigue syndrome – Depression – Insomnia Psychiatry (cont. ) – – – Panic disorders Schizophrenia Social Phobia Pulmonary and Allergy – – – Allergies Asthma Cough Cystic Fibrosis Food allergy Reproductive & Urologic – – – Benign prostatic enlargement Erectile dysfunction Premenstrual dysphoric disorder Sexual dysfunction in women Vulvar vestibulitis Urinary incontinence

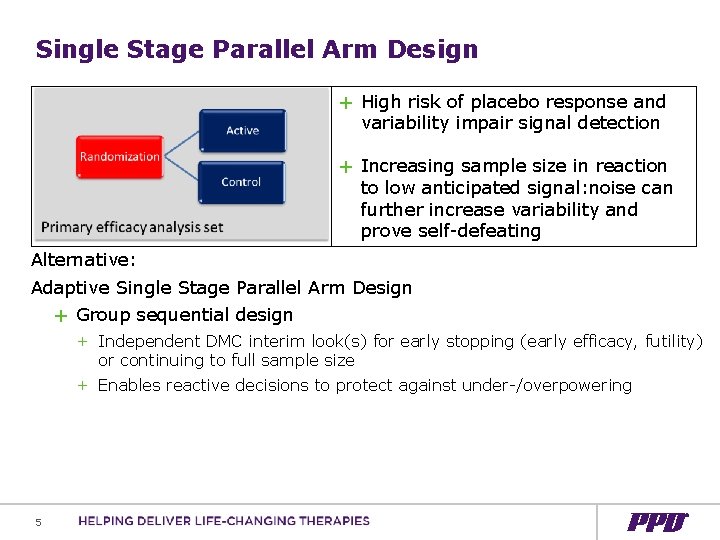
Single Stage Parallel Arm Design + High risk of placebo response and variability impair signal detection + Increasing sample size in reaction to low anticipated signal: noise can further increase variability and prove self-defeating Alternative: Adaptive Single Stage Parallel Arm Design + Group sequential design + Independent DMC interim look(s) for early stopping (early efficacy, futility) or continuing to full sample size + Enables reactive decisions to protect against under-/overpowering 5

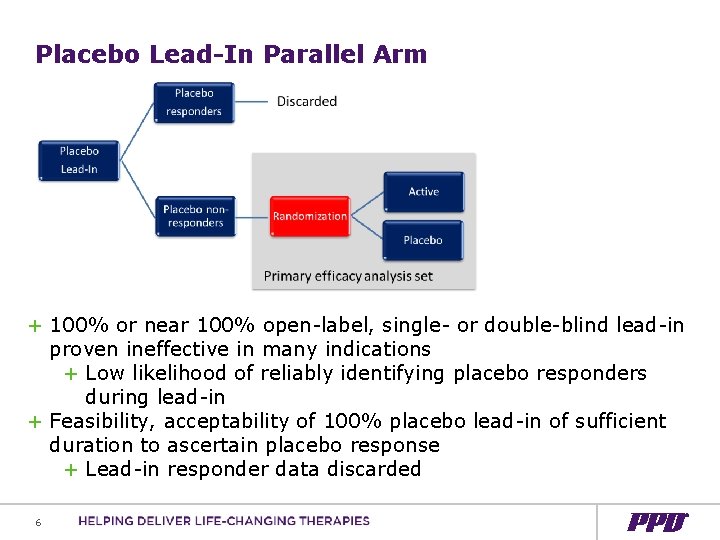
Placebo Lead-In Parallel Arm + 100% or near 100% open-label, single- or double-blind lead-in proven ineffective in many indications + Low likelihood of reliably identifying placebo responders during lead-in + Feasibility, acceptability of 100% placebo lead-in of sufficient duration to ascertain placebo response + Lead-in responder data discarded 6

Fava M et al 2003 Seminal Paper 7

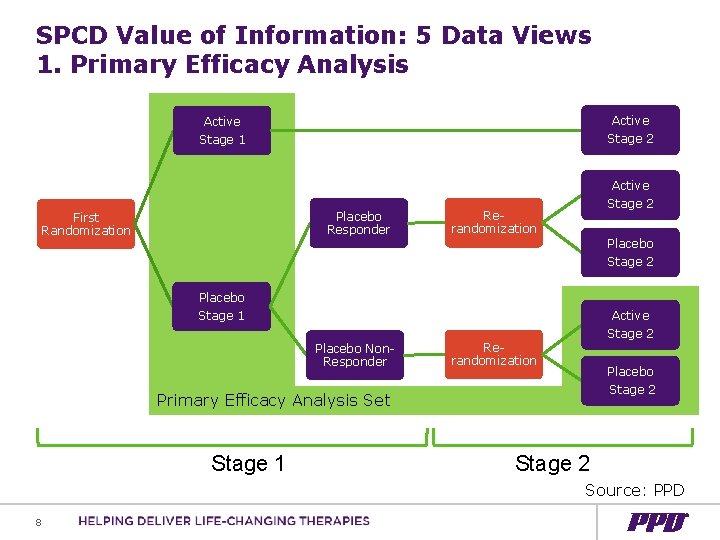
SPCD Value of Information: 5 Data Views 1. Primary Efficacy Analysis Active Stage 2 Active Stage 1 Placebo Responder First Randomization Active Stage 2 Rerandomization Placebo Stage 2 Placebo Stage 1 Placebo Non. Responder Active Stage 2 Rerandomization Placebo Stage 2 Primary Efficacy Analysis Set Stage 1 Stage 2 Source: PPD 8

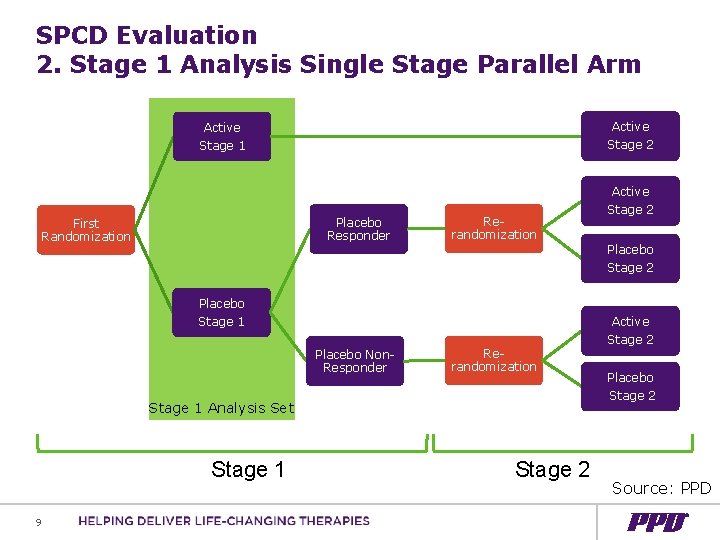
SPCD Evaluation 2. Stage 1 Analysis Single Stage Parallel Arm Active Stage 1 Stage 2 Active Placebo Responder First Randomization Rerandomization Placebo Stage 1 Placebo Non. Responder Rerandomization Stage 1 Analysis Set Stage 1 9 Stage 2 Placebo Stage 2 Active Stage 2 Placebo Stage 2 Source: PPD

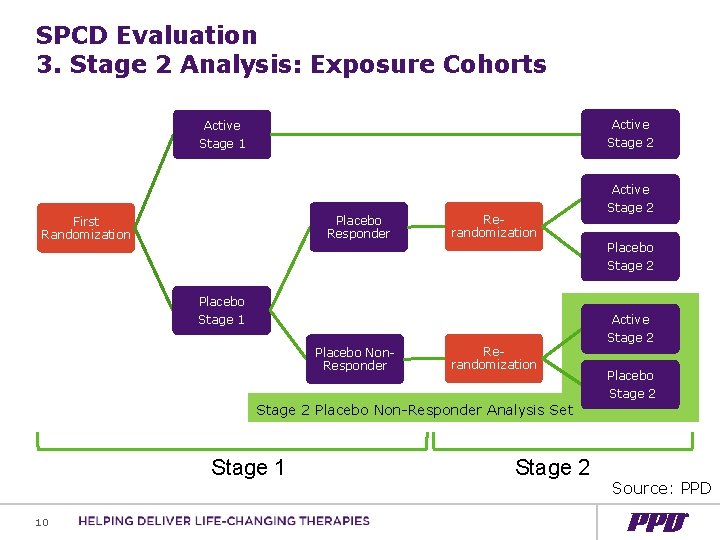
SPCD Evaluation 3. Stage 2 Analysis: Exposure Cohorts Active Stage 2 Active Stage 1 Active Placebo Responder First Randomization Rerandomization Placebo Stage 1 Placebo Non. Responder Rerandomization Stage 2 Placebo Stage 2 Active Stage 2 Placebo Non-Responder Analysis Set Stage 1 10 Stage 2 Source: PPD

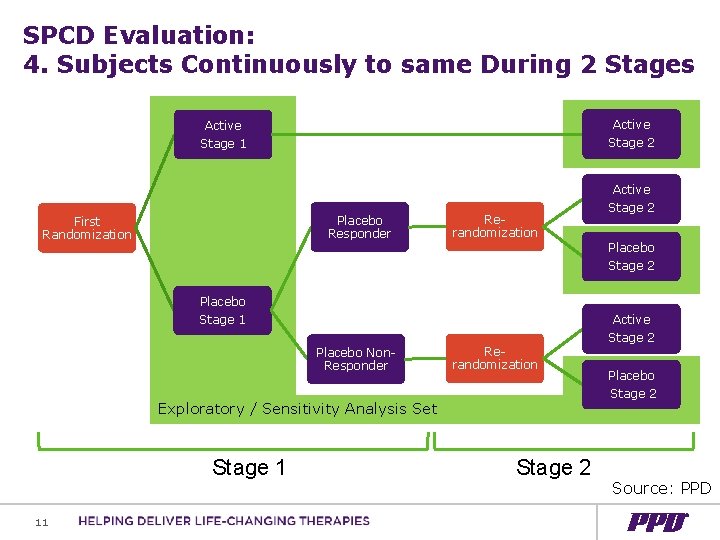
SPCD Evaluation: 4. Subjects Continuously to same During 2 Stages Active Stage 2 Active Stage 1 Active Placebo Responder First Randomization Rerandomization Placebo Stage 1 Placebo Non. Responder Rerandomization Exploratory / Sensitivity Analysis Set Stage 1 11 Stage 2 Placebo Stage 2 Active Stage 2 Placebo Stage 2 Source: PPD

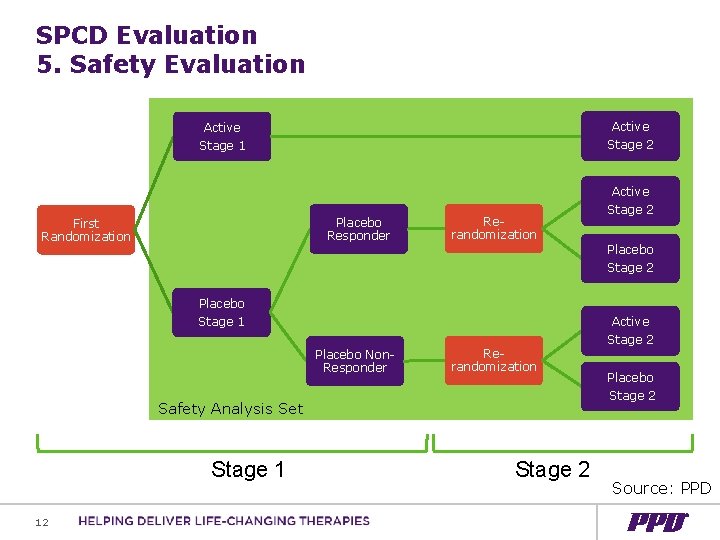
SPCD Evaluation 5. Safety Evaluation Active Stage 1 Stage 2 Active Placebo Responder First Randomization Rerandomization Placebo Stage 1 Placebo Non. Responder Rerandomization Safety Analysis Set Stage 1 12 Stage 2 Placebo Stage 2 Active Stage 2 Placebo Stage 2 Source: PPD

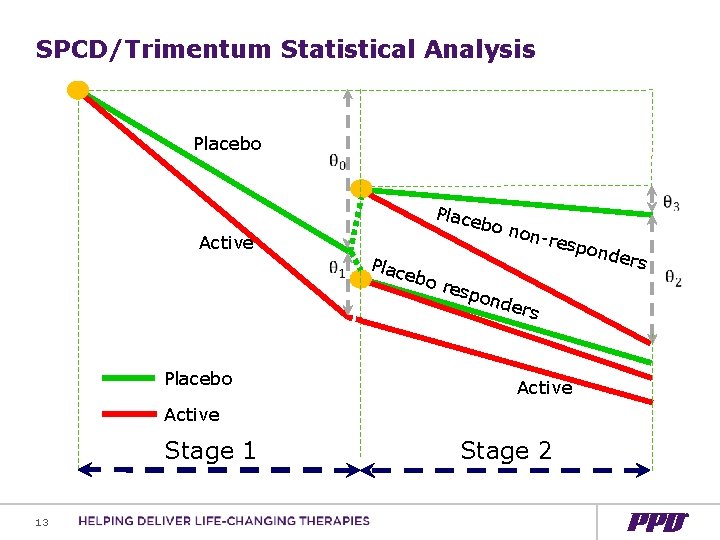
SPCD/Trimentum Statistical Analysis Placebo Place bo n Active on-re Plac ebo res spon d Placebo ers Active Stage 1 Stage 2 13 ers

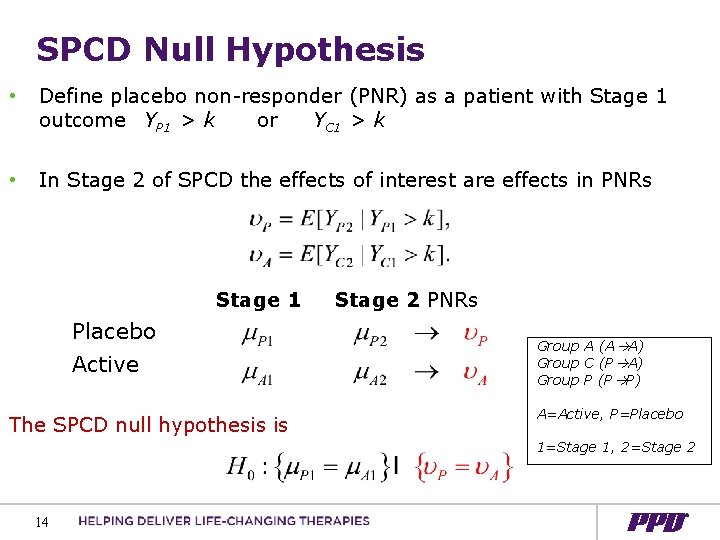
SPCD Null Hypothesis • Define placebo non-responder (PNR) as a patient with Stage 1 outcome YP 1 > k or YC 1 > k • In Stage 2 of SPCD the effects of interest are effects in PNRs Stage 1 Placebo Active The SPCD null hypothesis is Stage 2 PNRs Group A (A A) Group C (P A) Group P (P P) A=Active, P=Placebo 1=Stage 1, 2=Stage 2 14

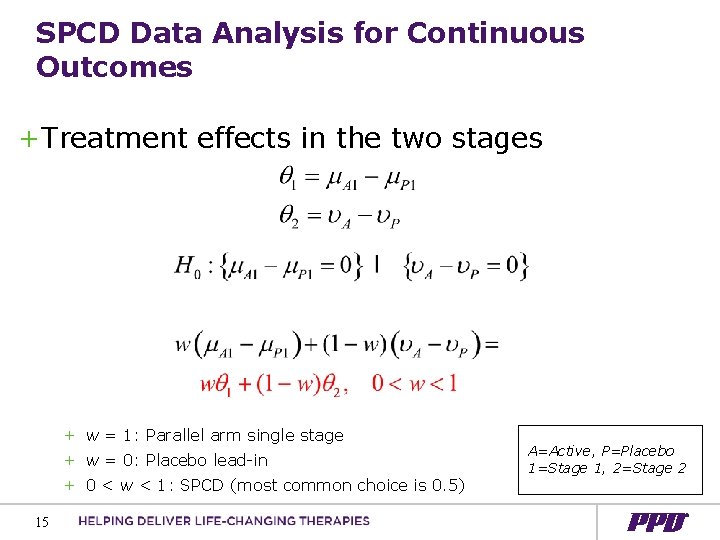
SPCD Data Analysis for Continuous Outcomes +Treatment effects in the two stages + w = 1: Parallel arm single stage + w = 0: Placebo lead-in + 0 < w < 1: SPCD (most common choice is 0. 5) 15 A=Active, P=Placebo 1=Stage 1, 2=Stage 2

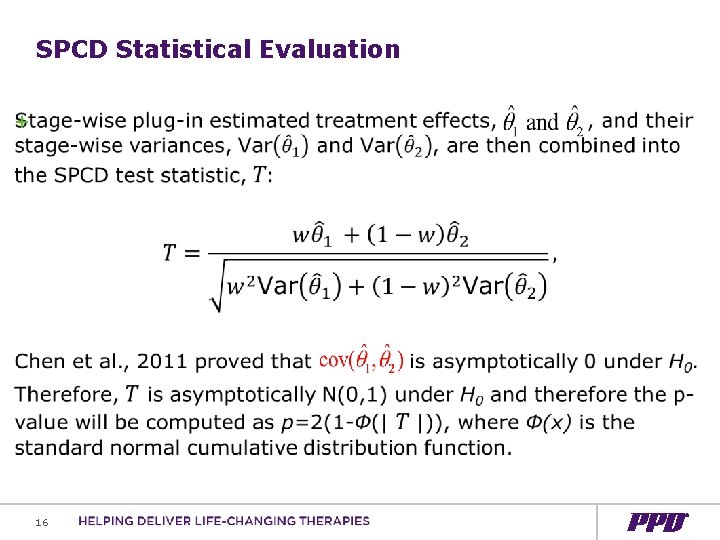
SPCD Statistical Evaluation + 16

Statistical Analysis Options for Continuous Endpoints: + Linear combination of estimated treatment effects: Tamura and Huang (2007) + MMRM: Yeh-Fong Chen et al. (2011) + Inverse normal combination of test statistics: Liu et al. (2012) + MMRM: Doros et al. (2013) Options for Binary Endpoints: + Linear combination of estimated treatment effects: Fava et al. (2003) + Score test: without covariates or other model terms (Huang & Tamura, 2010; Ivanova et al, 2011) + Logistic regression: with covariates or other model terms (draft publication by Silverman, Ivanova, and Fine, 2017 submitted publication). • 17 Missing data: dropouts typically included in analysis as non-responders

FDA's Chen YF et al CCT 2011 “the weighted test statistic based on MMRM estimates appears to be the most robust test statistic for SPD-Re. R in terms of type I error control, power performance, and estimation accuracy” 18

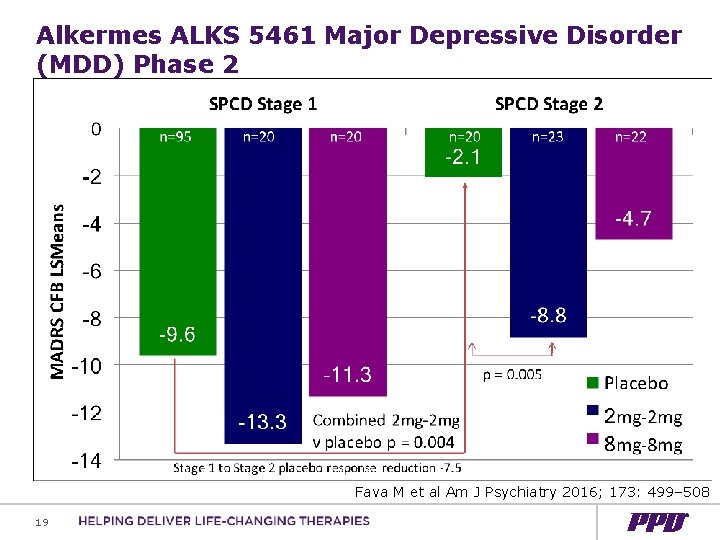
Alkermes ALKS 5461 Major Depressive Disorder (MDD) Phase 2 Fava M et al Am J Psychiatry 2016; 173: 499– 508 19

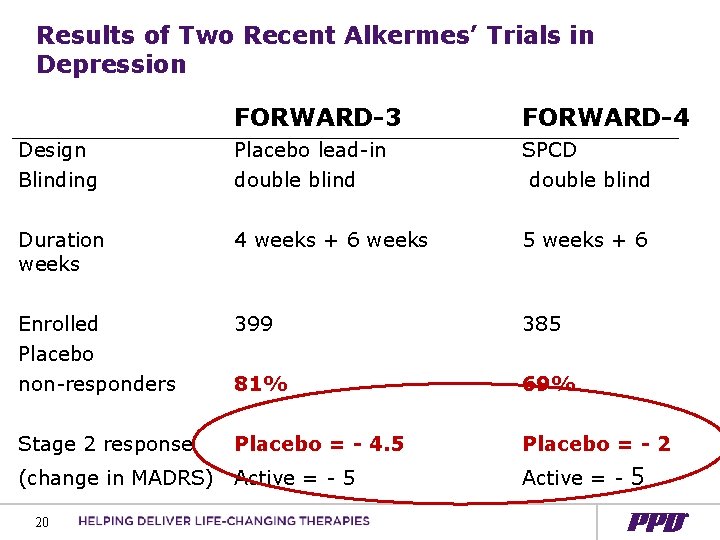
Results of Two Recent Alkermes’ Trials in Depression FORWARD-3 FORWARD-4 Design Blinding Placebo lead-in double blind SPCD double blind Duration weeks 4 weeks + 6 weeks 5 weeks + 6 Enrolled Placebo non-responders 399 385 81% 69% Stage 2 response Placebo = - 4. 5 Placebo = - 2 (change in MADRS) Active = - 5 20

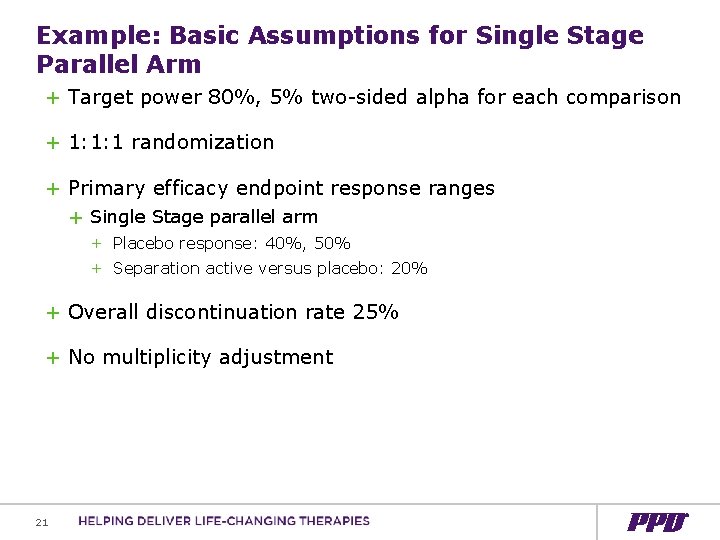
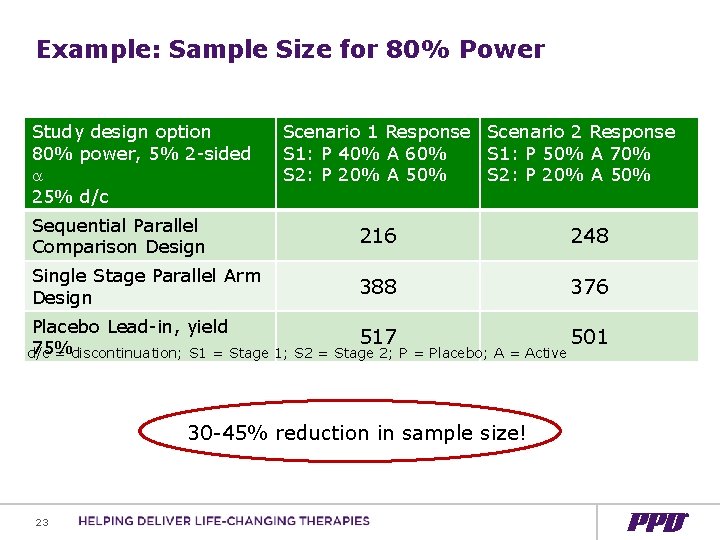
Example: Basic Assumptions for Single Stage Parallel Arm + Target power 80%, 5% two-sided alpha for each comparison + 1: 1: 1 randomization + Primary efficacy endpoint response ranges + Single Stage parallel arm + Placebo response: 40%, 50% + Separation active versus placebo: 20% + Overall discontinuation rate 25% + No multiplicity adjustment 21

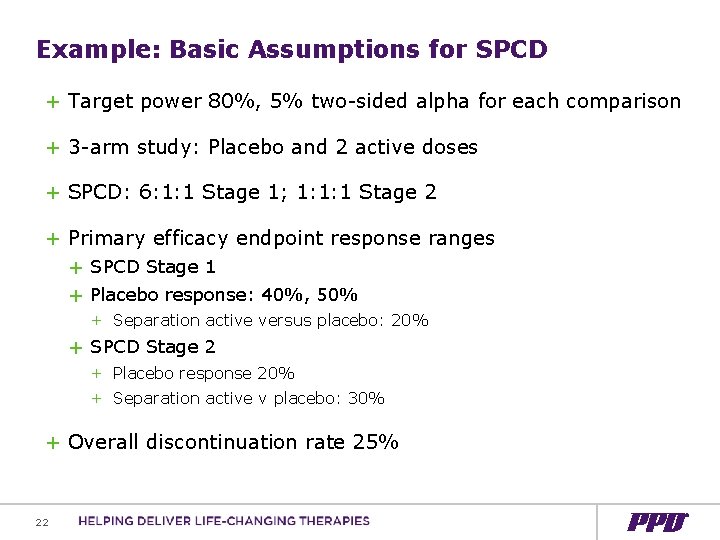
Example: Basic Assumptions for SPCD + Target power 80%, 5% two-sided alpha for each comparison + 3 -arm study: Placebo and 2 active doses + SPCD: 6: 1: 1 Stage 1; 1: 1: 1 Stage 2 + Primary efficacy endpoint response ranges + SPCD Stage 1 + Placebo response: 40%, 50% + Separation active versus placebo: 20% + SPCD Stage 2 + Placebo response 20% + Separation active v placebo: 30% + Overall discontinuation rate 25% 22

Example: Sample Size for 80% Power Study design option 80% power, 5% 2 -sided 25% d/c Scenario 1 Response Scenario 2 Response S 1: P 40% A 60% S 1: P 50% A 70% S 2: P 20% A 50% Sequential Parallel Comparison Design 216 248 Single Stage Parallel Arm Design 388 376 Placebo Lead-in, yield 517 501 75% d/c = discontinuation; S 1 = Stage 1; S 2 = Stage 2; P = Placebo; A = Active 30 -45% reduction in sample size! 23

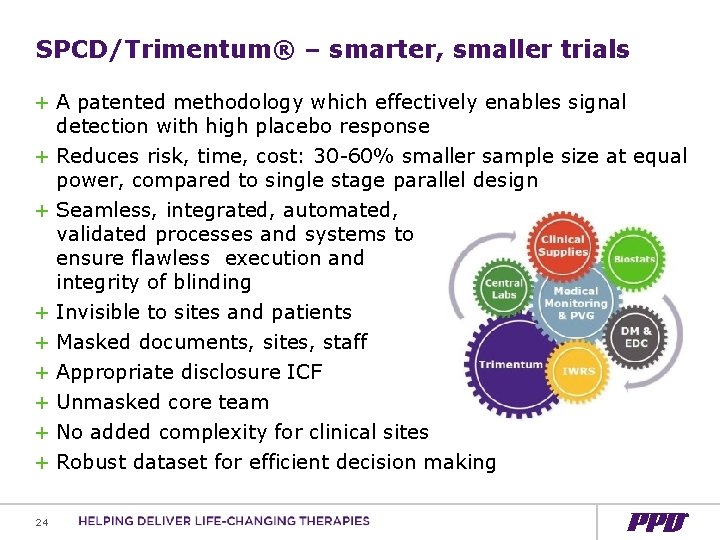
SPCD/Trimentum® – smarter, smaller trials + A patented methodology which effectively enables signal detection with high placebo response + Reduces risk, time, cost: 30 -60% smaller sample size at equal power, compared to single stage parallel design + Seamless, integrated, automated, validated processes and systems to ensure flawless execution and integrity of blinding + Invisible to sites and patients + Masked documents, sites, staff + Appropriate disclosure ICF + Unmasked core team + No added complexity for clinical sites + Robust dataset for efficient decision making 24

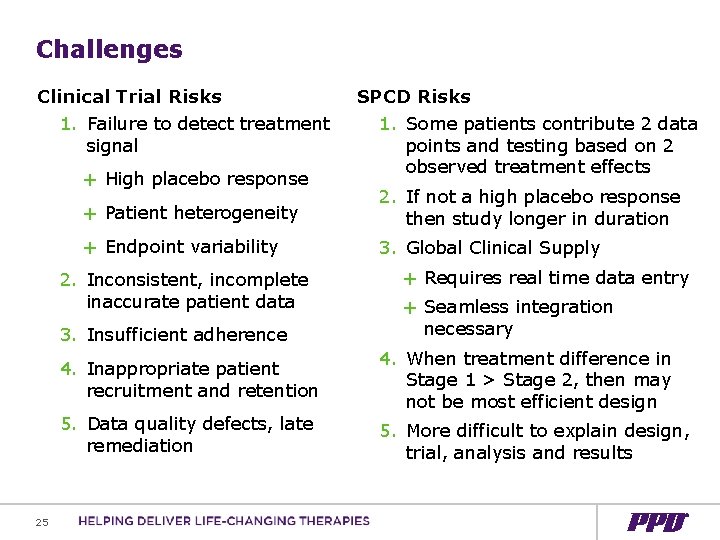
Challenges Clinical Trial Risks 1. Failure to detect treatment signal + High placebo response 1. Some patients contribute 2 data points and testing based on 2 observed treatment effects + Patient heterogeneity 2. If not a high placebo response then study longer in duration + Endpoint variability 3. Global Clinical Supply 2. Inconsistent, incomplete inaccurate patient data 3. Insufficient adherence 25 SPCD Risks + Requires real time data entry + Seamless integration necessary 4. Inappropriate patient recruitment and retention 4. When treatment difference in Stage 1 > Stage 2, then may not be most efficient design 5. Data quality defects, late remediation 5. More difficult to explain design, trial, analysis and results

Alternatives to SPCD Other solutions for Placebo Management: + Target appropriate population of interest with inclusion/exclusion criteria + Choose most appropriate instruments to measure efficacy + Reduce data variability in trial + Investigator & Site-facing staff selection/training on Placebo Management 26

References Brisonnet J. (translated by Hall H) Placebo Are You There? posted Feb 24 2015, https: //www. sciencebasedmedicine. org/placebo-are-you-there/ Chen Y. F. , Yang Y. , Hung H. , Wang S. Evaluation of performance of some enrichment designs dealing with high placebo response in psychiatric clinical trials. Contemporary Clinical Trials 2011; 32: 592 -604 Chi G YH, Li Y, Liu Y, Lewin D, Lim P. On Clinical Trials with a High Placebo Response Rate, Contemporary Clinical Trials. Communications 2016; 2: 34 -53. Doros G, Pencina M, Rybin D, Meisner A, Fava M. A repeated measures model for analysis of continuous outcomes in sequential parallel comparison design studies. Statistics in Medicine 2013; 32(16): 2767 -89. Fava, M, Schoenfeld, D. U. S. provisional Pat. App. No. 60/459, 517, filed March 31, 2003; U. S. Pat. App. No. 10/814, 852, filed March 31, 2004. Corresponding U. S. Patents include U. S. Pat. No. 7, 647, 235, issued January 12, 2010; U. S. Pat. No. 7, 840, 419, issued November 23, 2010; and U. S. Pat. Nos. 7, 983, 936; 8, 145, 504; 8, 145, 505; and 8, 219, 419. Fava M. , Evins A, Dorer D. , Schoenfeld D. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies and a novel study design approach. Psychotherapy and Psychosomatics 2003; 72: 115 -127. Fava, M, Mischoulon D, Iosifescu D, Witte J, Pencina M et al. A double-blind, placebo-controlled study of Aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychotherapy and Psychosomatics 2012; 81, 87 -97.

References Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, Du Y, Leigh-Pemberton R, Di. Petrillo L, Siverman B, Ehrich E. Opioid Modulation With Buprenorphine/Samidorphan as Adjuntctive Treatment for Inadequate Response to Antidepressants: A Randomized Double. Blind Placebo-Controlled Trial. Am J Psychiatry 2016; 173(5): 499– 508. Huang X. , Tamura R. Comparison of test statistics for the sequential parallel design. Statistics in Biopharmaceutical Research 2010; 2: 42 -50. Ivanova, A. , Qaqish, B. , Schoenfeld D. Sample size and power calculations for the sequential parallel design. Statistics in Medicine 2011; 30: 2793– 2803. Ivanova A, Qaqish BF. Basic models and parameter estimation in the sequential parallel comparison design with continuous outcomes (in preparation, 2017). Laughren TP. The scientific and ethical basis for placebo-controlled trials in depression and schizophrenia: an FDA perspective. European Psychiatry 2001; 16: 418 -423. Liu Q, Lim P, Singh J, Lewin D, Schwab B, Kent J. Doubly randomized delayed-start design for enrichment studies with responders or nonresponders. Journal of Biopharmaceutical Statistics. 2012; 22(4): 737 -57. Silverman RK, Ivanova A, and Fine J. 2017. (submitted publication) Tamura RN, Huang X. An examination of the efficiency of the sequential parallel design in psychiatric clinical trials. Clinical Trials: Journal of the Society of Clinical Trials 2007; 4: 309317. Trivedi MH, Rush AJ. Does a placebo run-in or placebo treatment cells affect the efficacy of antidepressant medications? Neuropsychopharmacology 1994; 11: 33 -43.

Disclaimer Copyright, 2017 by Pharmaceutical Product Development, LLC ("PPD"). This presentation, including the information contained herein and any associated commentary, ("Materials") is provided as a service of PPD. These Materials are based on publicly available information as well as the knowledge and experience of PPD's employees at time of drafting; however future accuracy cannot be guaranteed. As such, these Materials should not be solely relied upon without, or used as a substitute for, future consultation with PPD. Any further use of these Materials requires the express written consent of PPD. 29