SEPARATION OF MIXTURE TECHNIQUES These methods are by
SEPARATION OF MIXTURE TECHNIQUES • These methods are by physical means. • The chemical properties of each pure substance after separation are not changed. • The key is to find a physical property that one part of the mixture does have but the other doesn't.
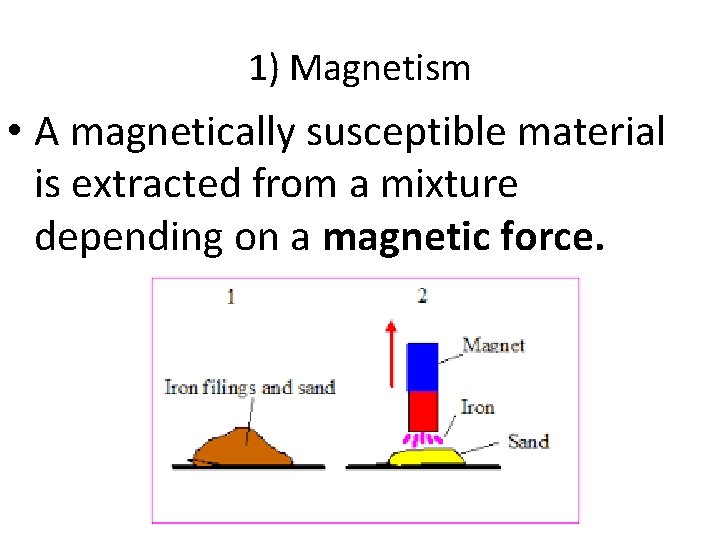
1) Magnetism • A magnetically susceptible material is extracted from a mixture depending on a magnetic force.
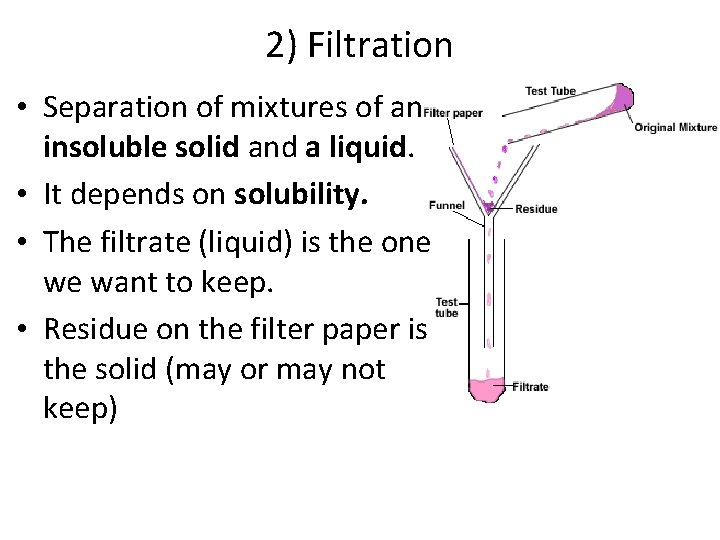
2) Filtration • Separation of mixtures of an insoluble solid and a liquid. • It depends on solubility. • The filtrate (liquid) is the one we want to keep. • Residue on the filter paper is the solid (may or may not keep)
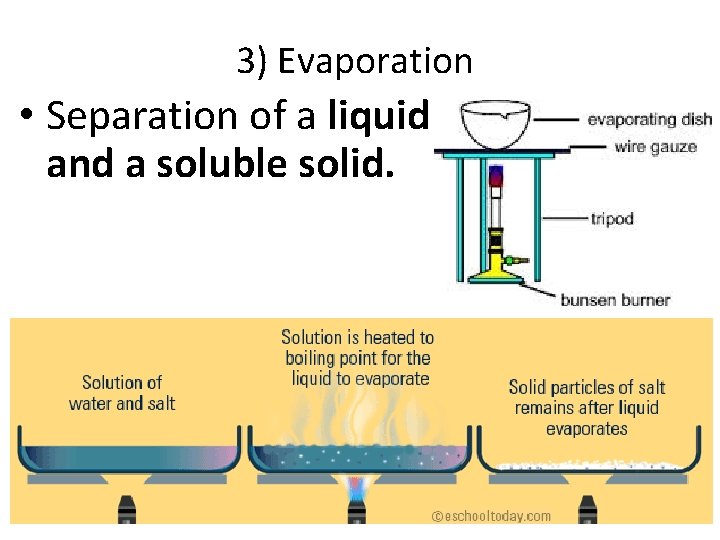
3) Evaporation • Separation of a liquid and a soluble solid.

4) Decanting • Used to separate a liquid from an insoluble solid. The solid that stays in the bottom is the one we want to keep.
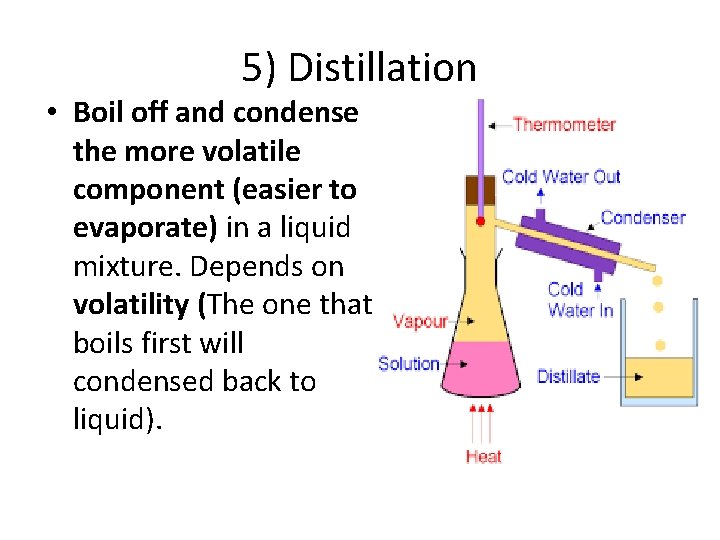
5) Distillation • Boil off and condense the more volatile component (easier to evaporate) in a liquid mixture. Depends on volatility (The one that boils first will condensed back to liquid).
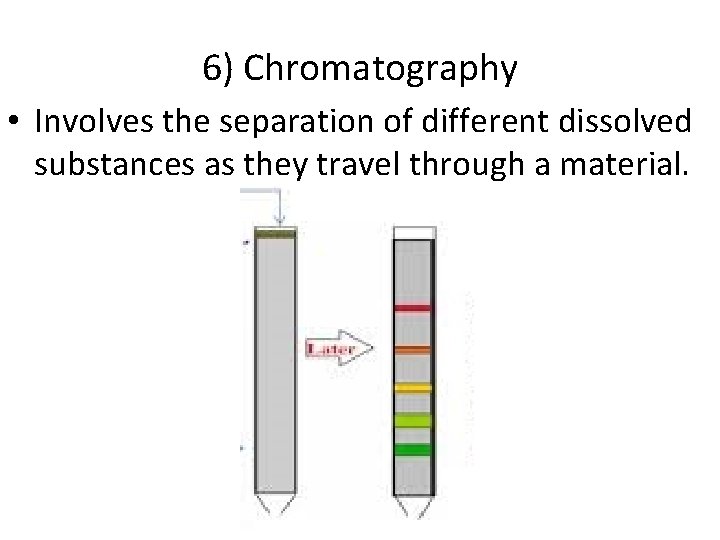
6) Chromatography • Involves the separation of different dissolved substances as they travel through a material.
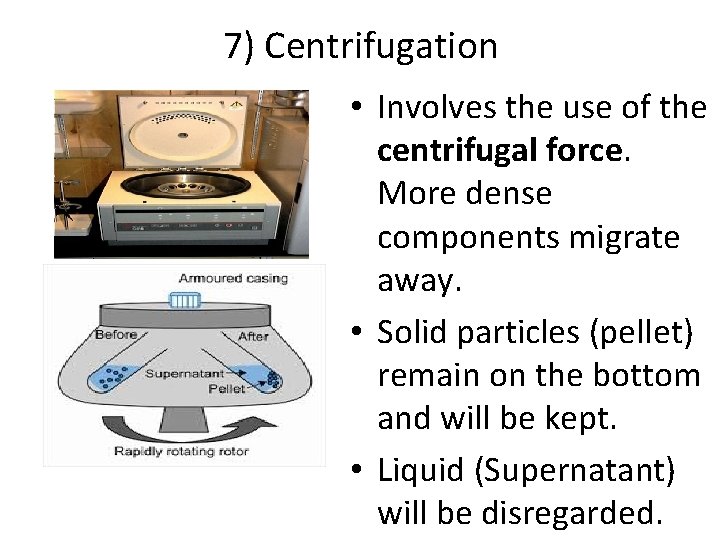
7) Centrifugation • Involves the use of the centrifugal force. More dense components migrate away. • Solid particles (pellet) remain on the bottom and will be kept. • Liquid (Supernatant) will be disregarded.
- Slides: 8