Separation and Purification of Proteins Protein Fractionation Definition






- Slides: 19

Separation and Purification of Proteins

Protein Fractionation Definition Protein Fractionation is a process or series of processes intended to isolate a single or multiple type of protein from a complex mixture of proteins. 6

Fractionation of food Fractionation is also used for culinary purposes, as coconut oil and palm oil are fractionated to produce oils of different viscosities, that may be used for different purposes. Mango oil is an oil fraction obtained during the processing of mango butter. Milk can also be fractionated to recover the milk protein concentrate or the milk basic proteins fraction.

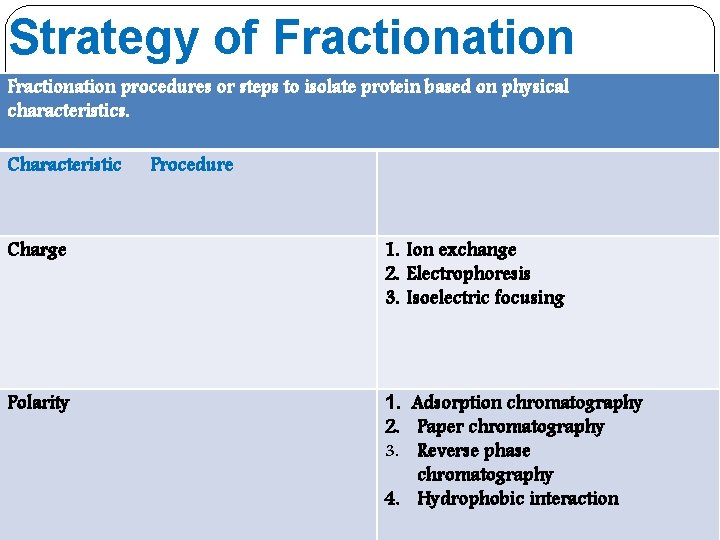
Strategy of Fractionation procedures or steps to isolate protein based on physical characteristics. Characteristic Procedure Charge 1. Ion exchange 2. Electrophoresis 3. Isoelectric focusing Polarity 1. Adsorption chromatography 2. Paper chromatography 3. Reverse phase chromatography 4. Hydrophobic interaction

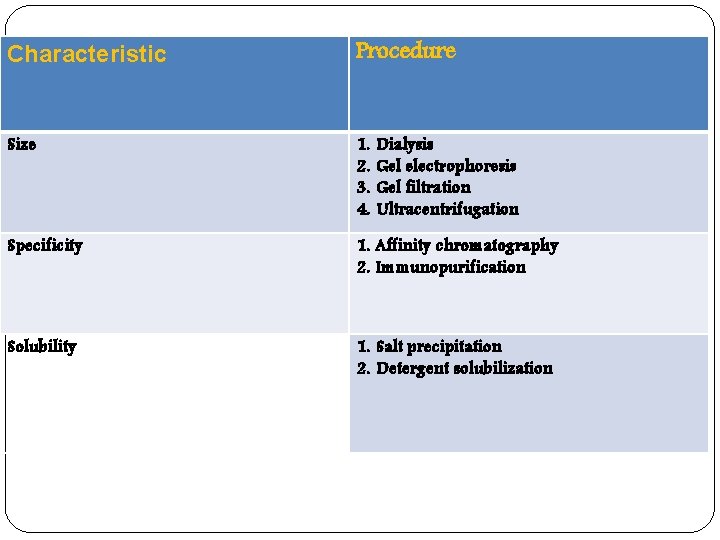
Characteristic Procedure Size 1. 2. 3. 4. Specificity 1. Affinity chromatography 2. Immunopurification Solubility 1. Salt precipitation 2. Detergent solubilization Dialysis Gel electrophoresis Gel filtration Ultracentrifugation

Protein Assay Lowry method as being the most accurate and sensitive method down to 0. 01 mg/m. L and widely used. Based on the biuret reaction in which the peptide bonds of the proteins react with copper under alkaline conditions to produce CU+, which reacts with the Follins reagent resulting in strong blue colour which depends partly on aromatic a. acid such as tyrosine and tryptophane.

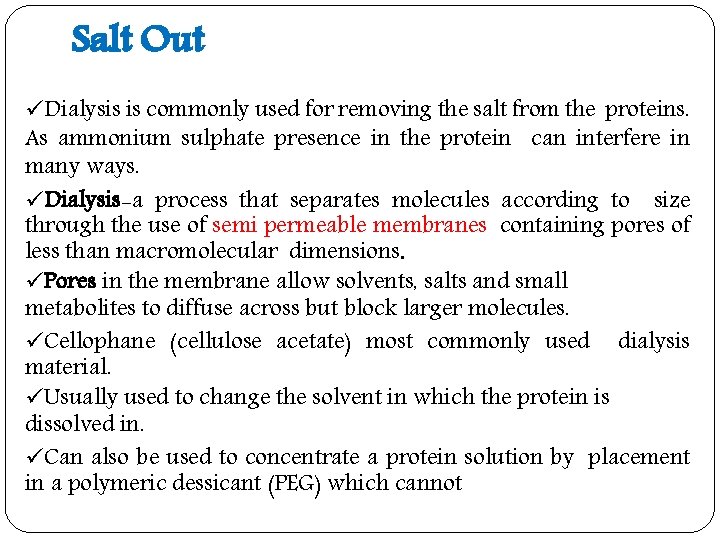
Salt Out üDialysis is commonly used for removing the salt from the proteins. As ammonium sulphate presence in the protein can interfere in many ways. üDialysis-a process that separates molecules according to size through the use of semi permeable membranes containing pores of less than macromolecular dimensions. üPores in the membrane allow solvents, salts and small metabolites to diffuse across but block larger molecules. üCellophane (cellulose acetate) most commonly used dialysis material. üUsually used to change the solvent in which the protein is dissolved in. üCan also be used to concentrate a protein solution by placement in a polymeric dessicant (PEG) which cannot

Figure : Use of dialysis to separate small and large molecules.

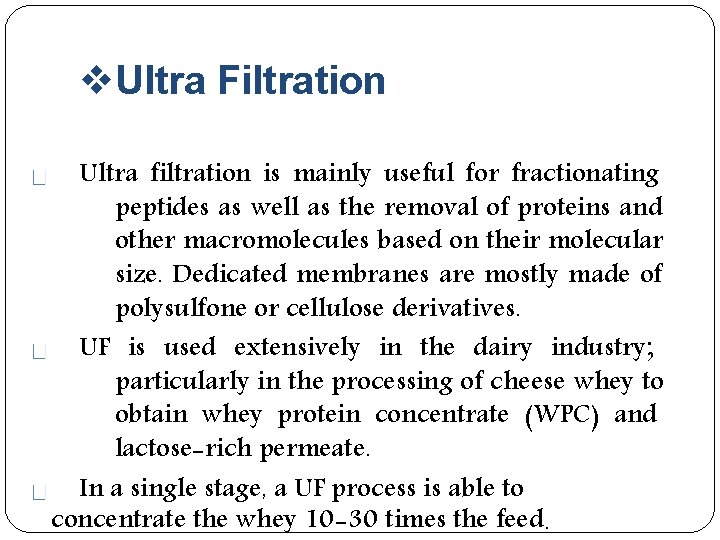
Ultra Filtration Ultra filtration is mainly useful for fractionating peptides as well as the removal of proteins and other macromolecules based on their molecular size. Dedicated membranes are mostly made of polysulfone or cellulose derivatives. UF is used extensively in the dairy industry; particularly in the processing of cheese whey to obtain whey protein concentrate (WPC) and lactose-rich permeate. In a single stage, a UF process is able to concentrate the whey 10 -30 times the feed.

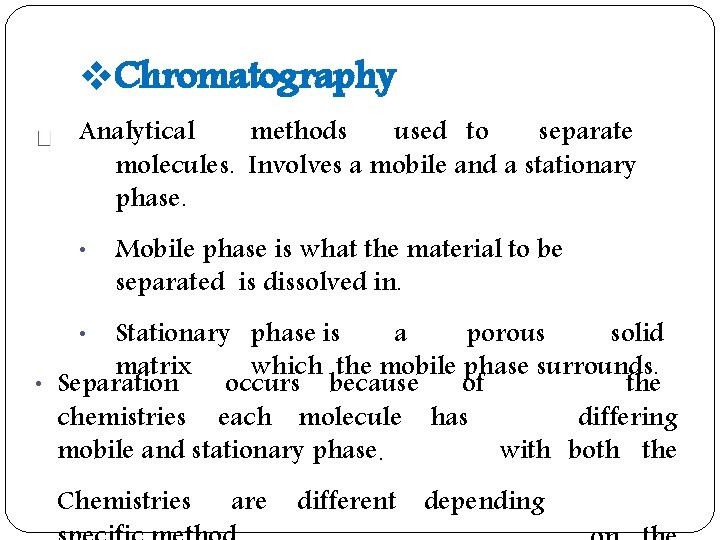
Chromatography Analytical methods used to separate molecules. Involves a mobile and a stationary phase. • Mobile phase is what the material to be separated is dissolved in. Stationary phase is a porous solid matrix which the mobile phase surrounds. • Separation occurs because the of chemistries each molecule has differing with both the mobile and stationary phase. • Chemistries are different depending

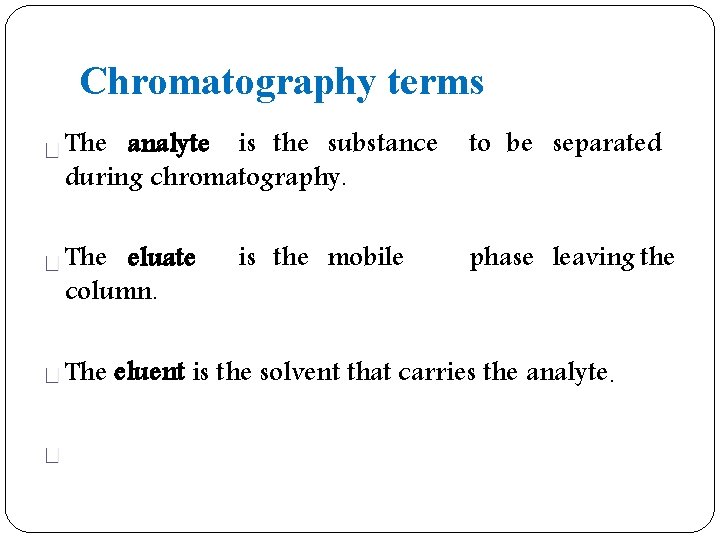
Chromatography terms The analyte is the substance during chromatography. to be separated The eluate column. phase leaving the is the mobile The eluent is the solvent that carries the analyte.

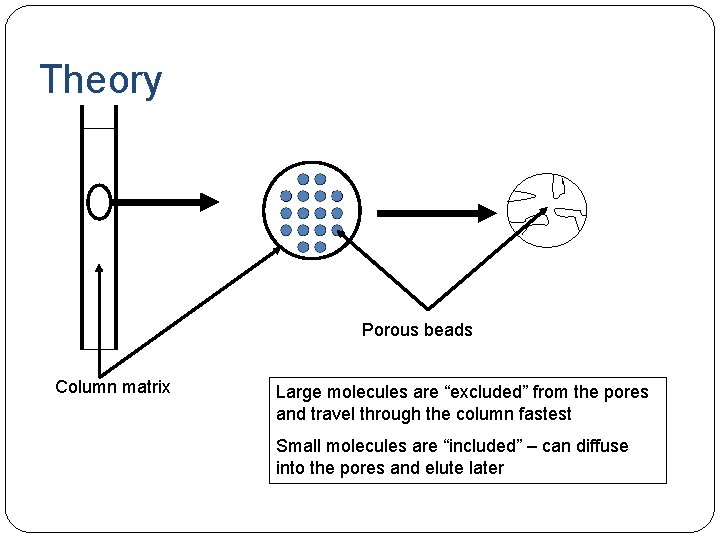
Gel Filtration Chromatography Gel filtration (chromatography), is also known as molecular sieve chromatography. Gel filtration chromatography separates molecules according to their size and shape. The stationary phase consists of beads containing pores that span a relatively narrow size range. Smaller molecules spend more time inside the beads than larger molecules and therefore elute later (after a larger volume of mobile phase has passed through the column).

Theory Porous beads Column matrix Large molecules are “excluded” from the pores and travel through the column fastest Small molecules are “included” – can diffuse into the pores and elute later

Thin Layer Chromatography

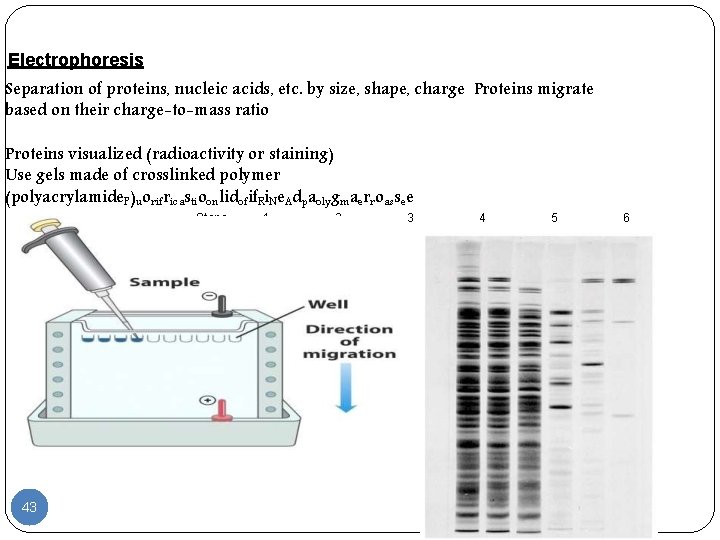
Electrophoresis Methods Electrophoresis separates mixtures of proteins based on charge, charge/mass ratio or size. Principle 1. It is the process of moving charged biomolecules in solution by applying an electrical field across the mixture. 2. Biomolecules moved with a speed dependent on their charge, shape, and size and separation occures on the basis of molecular size. 41

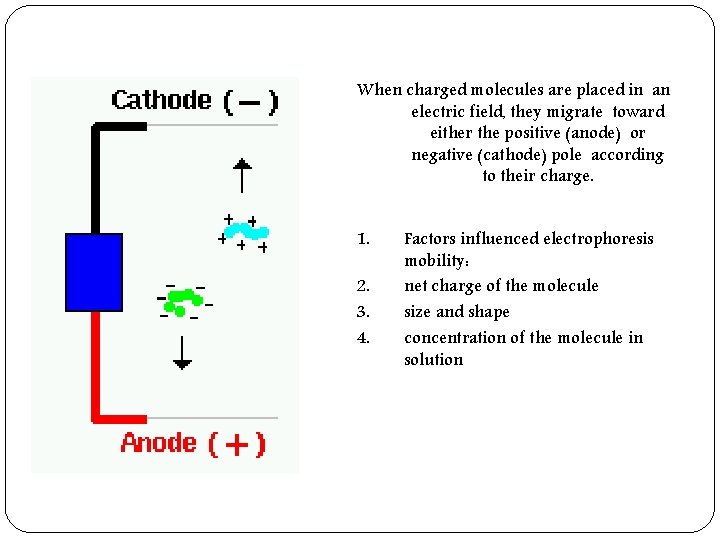
When charged molecules are placed in an electric field, they migrate toward either the positive (anode) or negative (cathode) pole according to their charge. 1. 2. 3. 4. Factors influenced electrophoresis mobility: net charge of the molecule size and shape concentration of the molecule in solution

Electrophoresis Separation of proteins, nucleic acids, etc. by size, shape, charge Proteins migrate based on their charge-to-mass ratio Proteins visualized (radioactivity or staining) Use gels made of crosslinked polymer (polyacrylamide. P)uorifricastioonlidofif. Ri. Ne. Adpaolygmaerroassee Steps 43 1 2 3 4 5 6

References http: //www. google. com/search? q=affinity+chromatogr aphy+%3 B 793%3 B 528 http: //www. google. com/webhp? nord=1#nord=1&q=el ectrophoresis http: //www. sigmaaldrich. com/catalog/product/sigma/d 9938? lang=en®ion=IN Sawhney S. K. and Randhir Singh (2011). Intoductory Practical Biochemistry. 9 th Reprint pp 195 -216.
