Separating Mixtures Filtration Uses a porous barrier to

Separating Mixtures: Filtration: Uses a porous barrier to separate a solid (undissolved) from a liquid. Examples: Sunflower seeds and water. Black pepper and water. Solid wastes and water.
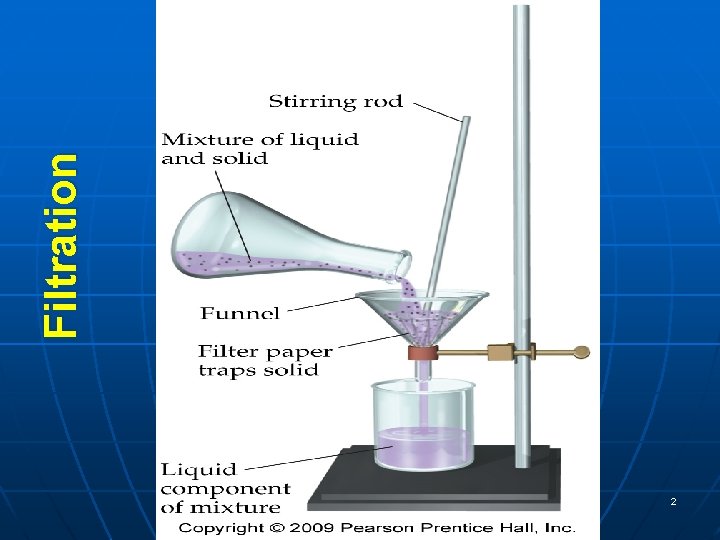
Filtration Tro's "Introductory Chemistry", Chapter 3 2

Separating Mixtures: Decantation: To separate immiscible liquids (different densities) or a solid which is settled at the bottom of a liquid. Examples: Sand & water. Small rocks & and water. Oil and water. Diesel & water. Oil & vinegar. Use a separatory funnel.

Separatory funnel.
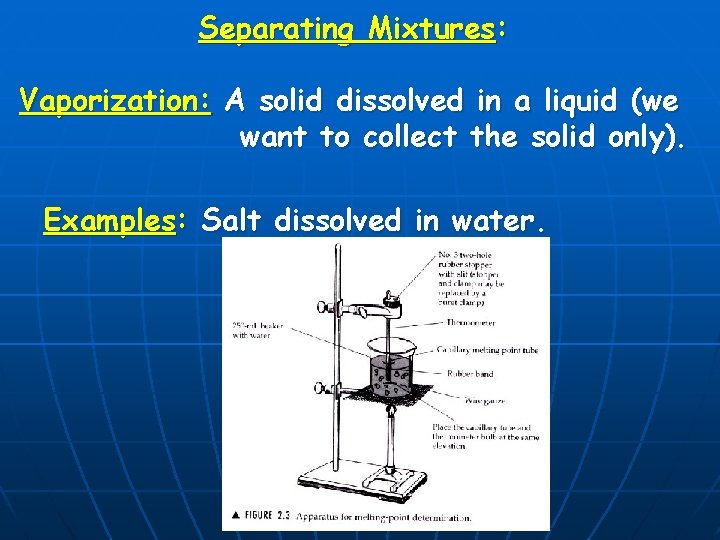
Separating Mixtures: Vaporization: A solid dissolved in a liquid (we want to collect the solid only). Examples: Salt dissolved in water.

Separating Mixtures: Distillation: Is based on differences in the boiling points (two liquids or liquid + solid dissolved) and you want to recover both. Examples: Salt dissolved in water. Alcohol dissolved in water.
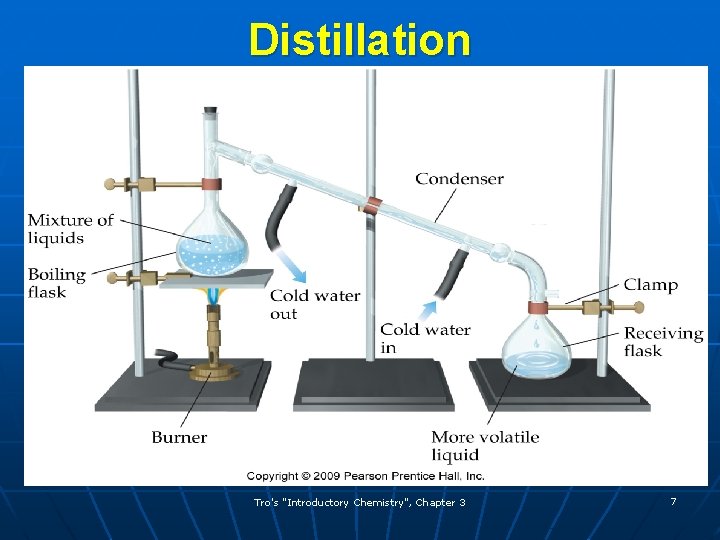
Distillation Tro's "Introductory Chemistry", Chapter 3 7
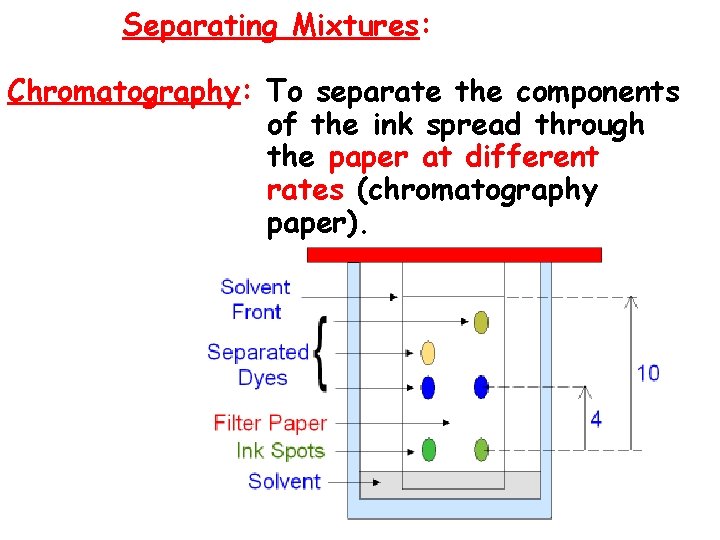
Separating Mixtures: Chromatography: To separate the components of the ink spread through the paper at different rates (chromatography paper).
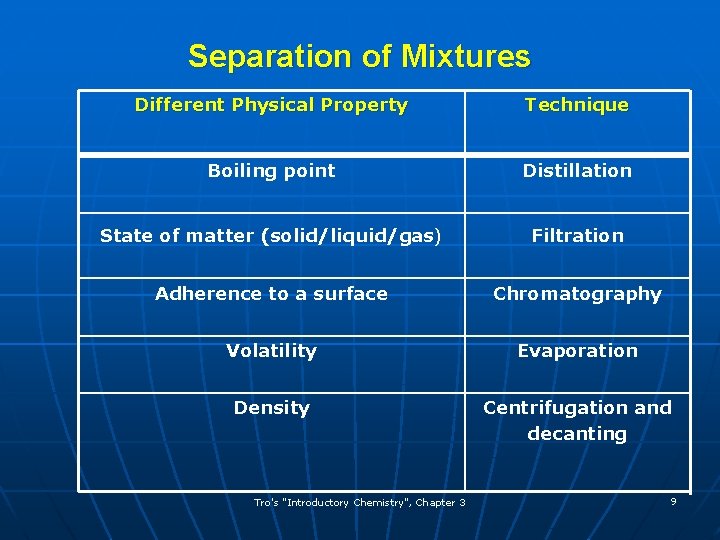
Separation of Mixtures Different Physical Property Technique Boiling point Distillation State of matter (solid/liquid/gas) Filtration Adherence to a surface Chromatography Volatility Evaporation Density Centrifugation and decanting Tro's "Introductory Chemistry", Chapter 3 9
- Slides: 9