Separating Azeotropic Mixtures CHEN 4460 Process Synthesis Simulation






- Slides: 21

Separating Azeotropic Mixtures CHEN 4460 – Process Synthesis, Simulation and Optimization Dr. Mario Richard Eden Department of Chemical Engineering Auburn University Lecture No. 6 – Review of Non-ideal Thermodynamics September 25, 2012 Contains Material Developed by Dr. Daniel R. Lewin, Technion, Israel

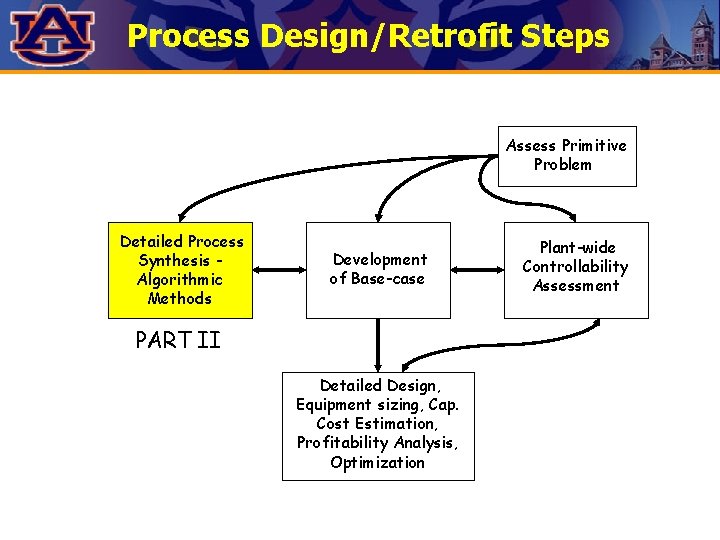
Process Design/Retrofit Steps Assess Primitive Problem Detailed Process Synthesis Algorithmic Methods Development of Base-case PART II Detailed Design, Equipment sizing, Cap. Cost Estimation, Profitability Analysis, Optimization Plant-wide Controllability Assessment

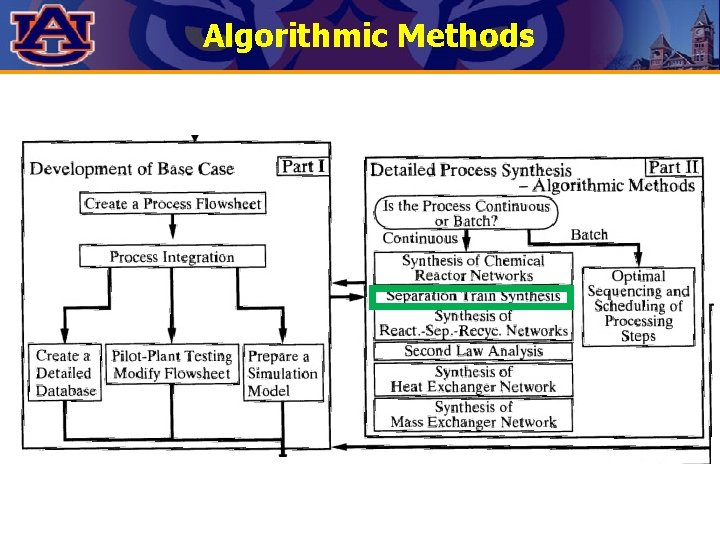
Algorithmic Methods

Lecture 6 – Introduction • Separation sequences are complicated by the presence of azeotropes, often involving mixtures of oxygenated organic compounds: Alcohols Ketones Ethers Acids Water • In these cases, distillation boundaries limit the product compositions of a column to lie within a bounded region. • This prevents the removal of certain species in high concentrations.

Lecture 6 – Objectives Be able to sketch the residue curves on a ternary phase diagram Be able to define the range of possible product compositions using distillation, given the feed composition and the ternary phase diagram

Basics: The Lever Rule

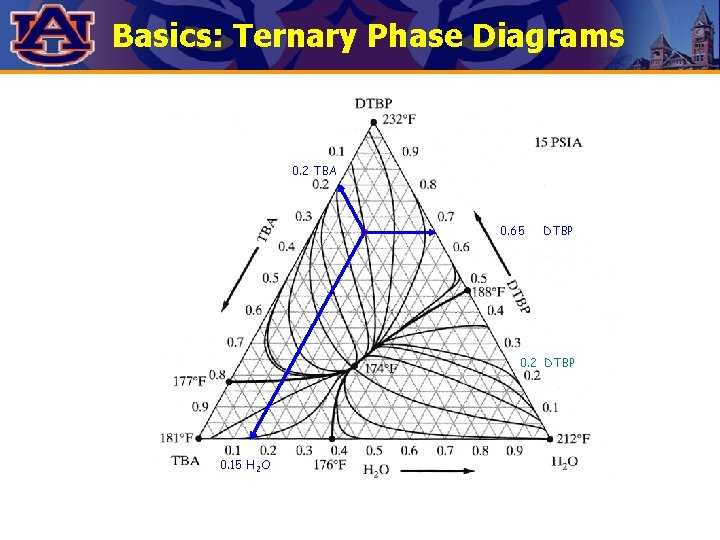
Basics: Ternary Phase Diagrams 0. 2 TBA 0. 65 DTBP 0. 2 DTBP 0. 15 H 2 O

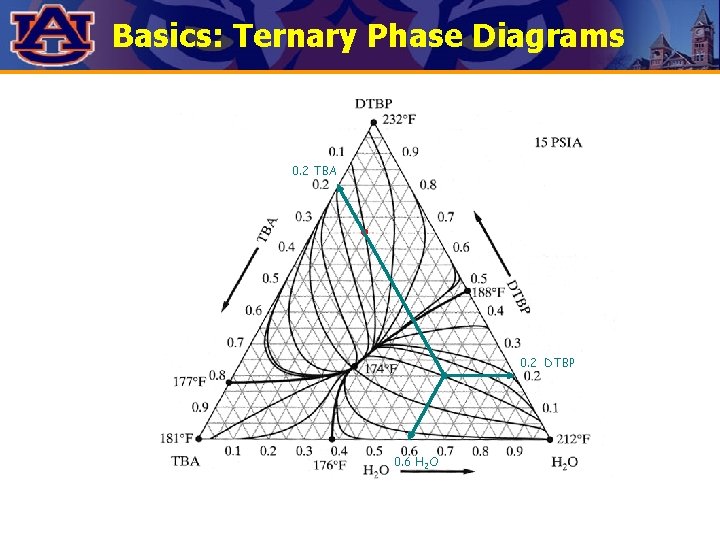
Basics: Ternary Phase Diagrams 0. 2 TBA 0. 2 DTBP 0. 6 H 2 O

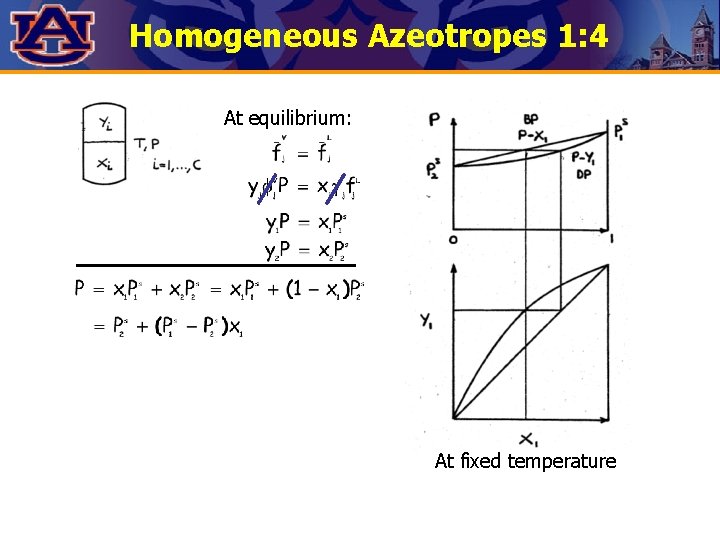
Homogeneous Azeotropes 1: 4 At equilibrium: At fixed temperature

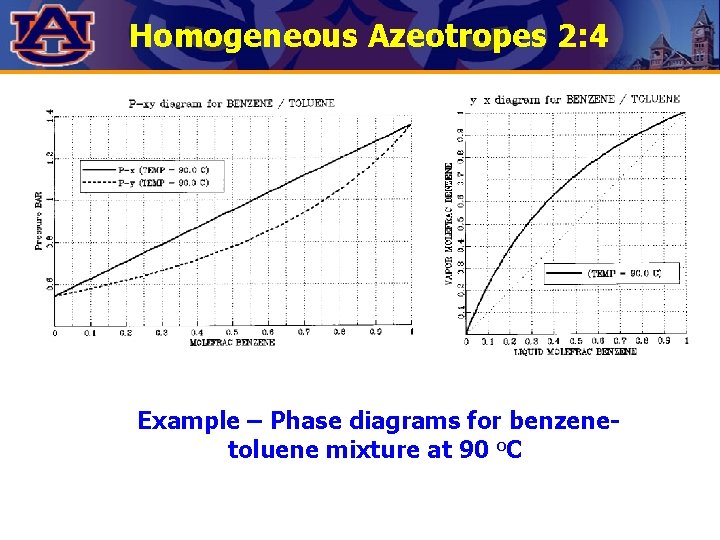
Homogeneous Azeotropes 2: 4 Example – Phase diagrams for benzenetoluene mixture at 90 o. C

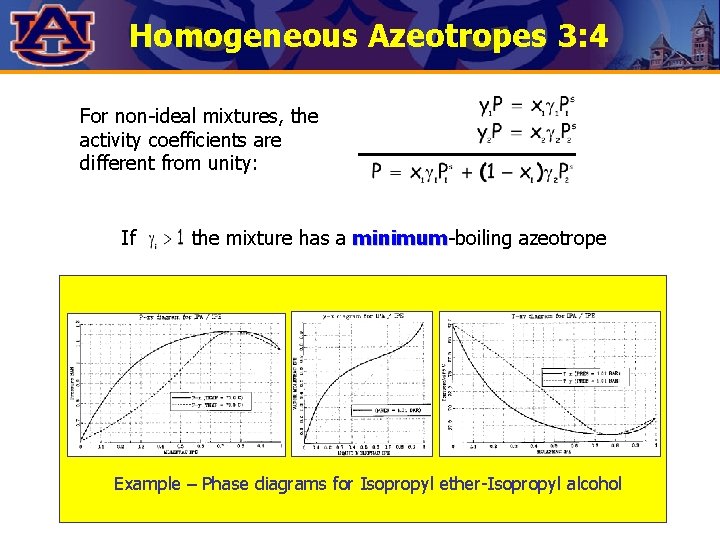
Homogeneous Azeotropes 3: 4 For non-ideal mixtures, the activity coefficients are different from unity: If the mixture has a minimum-boiling azeotrope minimum Example – Phase diagrams for Isopropyl ether-Isopropyl alcohol

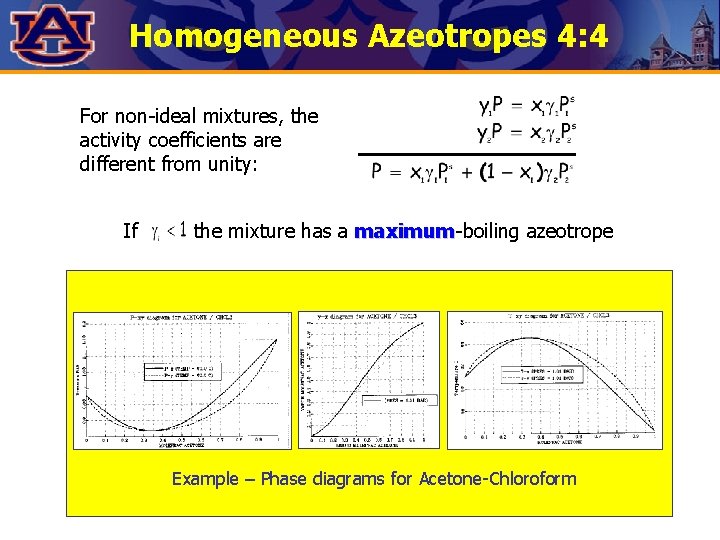
Homogeneous Azeotropes 4: 4 For non-ideal mixtures, the activity coefficients are different from unity: If the mixture has a maximum-boiling azeotrope maximum Example – Phase diagrams for Acetone-Chloroform

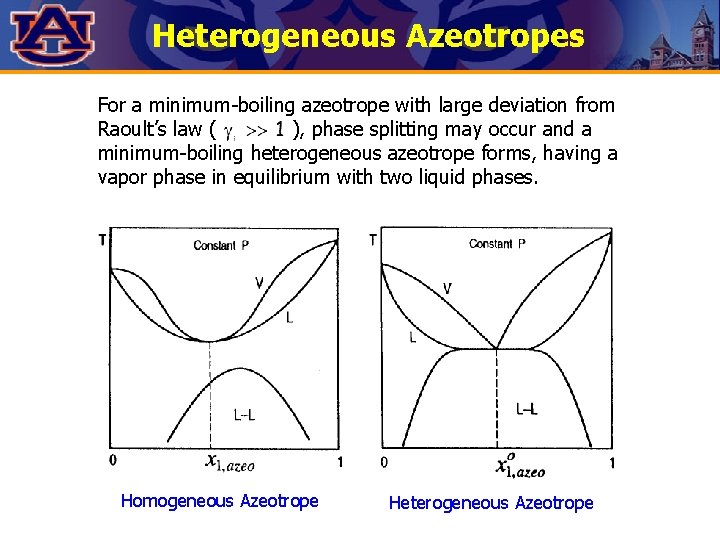
Heterogeneous Azeotropes For a minimum-boiling azeotrope with large deviation from Raoult’s law ( ), phase splitting may occur and a minimum-boiling heterogeneous azeotrope forms, having a vapor phase in equilibrium with two liquid phases. Homogeneous Azeotrope Heterogeneous Azeotrope

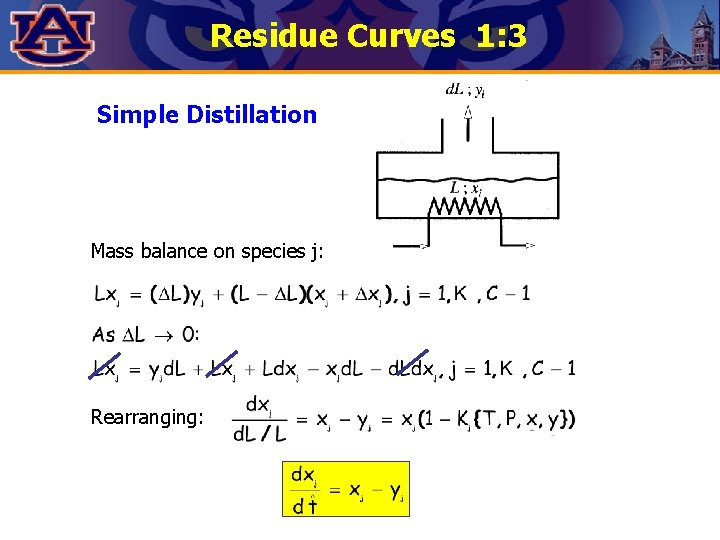
Residue Curves 1: 3 Simple Distillation Mass balance on species j: Rearranging:

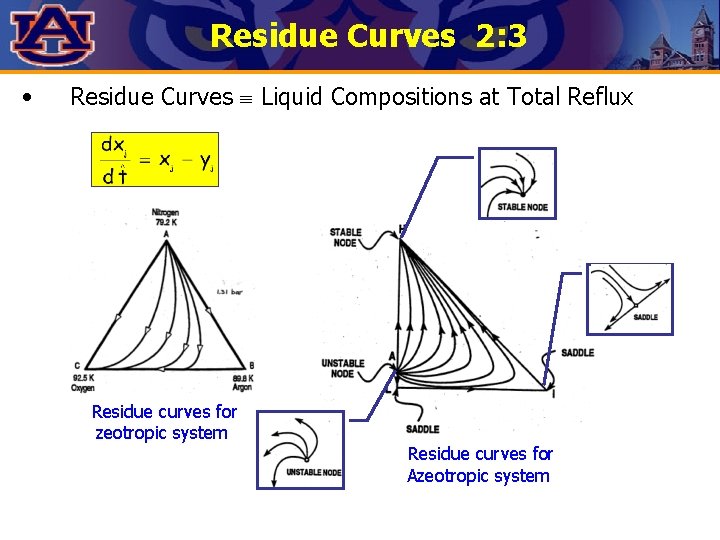
Residue Curves 2: 3 • Residue Curves Liquid Compositions at Total Reflux Residue curves for zeotropic system Residue curves for Azeotropic system

Residue Curves 3: 3 • Residue Curves Liquid Compositions at Total Reflux Species balance on top n-1 trays: Approximation for liquid phase: Substituting: Rectifying section of distillation column At total reflux, D = 0 and Vn = Ln-1

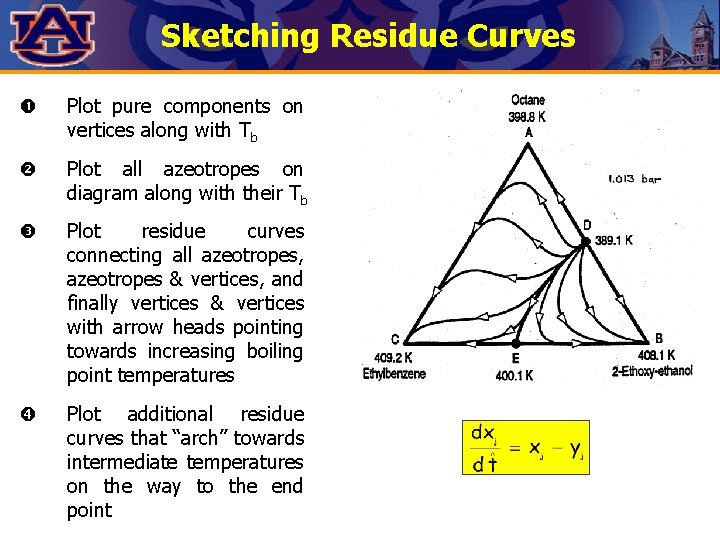
Sketching Residue Curves Plot pure components on vertices along with Tb Plot all azeotropes on diagram along with their Tb Plot residue curves connecting all azeotropes, azeotropes & vertices, and finally vertices & vertices with arrow heads pointing towards increasing boiling point temperatures Plot additional residue curves that “arch” towards intermediate temperatures on the way to the end point

Product Compositions Regions • For zeotropic systems – L: Lowest boiling component, I: Intermediate boiling component, H: Highest boiling component, F: Feed composition Pure L distillate Pure H bottoms

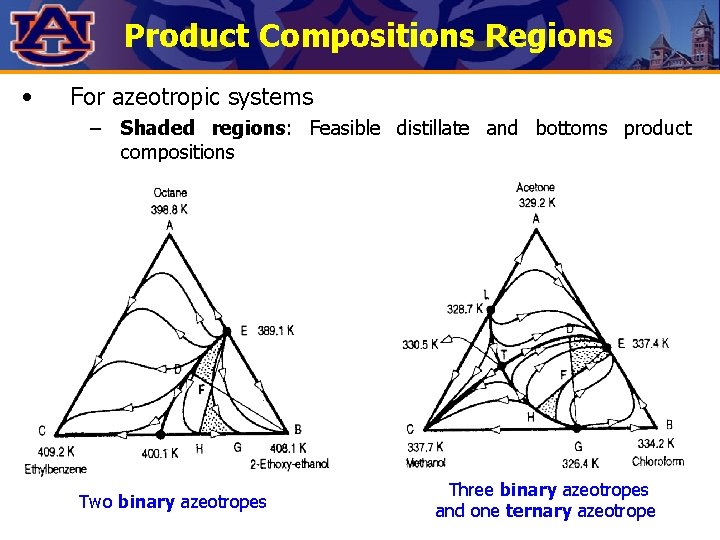
Product Compositions Regions • For azeotropic systems – Shaded regions: Feasible distillate and bottoms product compositions Two binary azeotropes Three binary azeotropes and one ternary azeotrope

Summary – Non-ideal Thermo On completion of this part, you should: Be able to sketch the residue curves on a ternary phase diagram Be able to define the range of possible product compositions using distillation, given the feed composition and the ternary phase diagram

Other Business • Homework – – • SSLW: 8. 14 b-d, 8. 15 Due Tuesday October 2 Next Lecture (October 2) – – • Part 1: Sequencing Azeotropic Distillation Columns (SSLW 230 -251) Part 2: Review for Midterm Exam – – October 9 during lecture Open book or closed book?