Sensitivity of DNA Looping to Sequencedependent Stiffness Sachin
![Formulation of Nonlinear Rod Dynamics [Goyal et al. , 2005] Field Variables: {v, ω, Formulation of Nonlinear Rod Dynamics [Goyal et al. , 2005] Field Variables: {v, ω,](https://slidetodoc.com/presentation_image_h2/04646844dfb517ea2ec6e5f5ae5c750e/image-6.jpg)

- Slides: 16

Sensitivity of DNA Looping to Sequence-dependent Stiffness Sachin Goyal 1, Todd Lillian 2, David Wilson 2, Edgar Meyhofer 2, Jens-Christian Meiners 2 and Noel Perkins 2. 1 Woods Hole Oceanographic Institution, Woods Hole, MA, USA, 2 University of Michigan, Ann Arbor, MI, USA. Protein-mediated DNA looping plays an important role in gene regulation. Empirically it is known that sequence-dependent mechanical effect such as intrinsic bends or softer regions in the substrate DNA affect loop formation, but quantitative models are lacking. We employ a continuum rod model to simulate protein-mediated DNA looping as a means to explore how the sequence maps to the overall structural properties of the duplex. The model includes sequence-dependent intrinsic curvature, chirality, and stiffness. We address the fundamental question of how sequence-dependent stiffness influences the looping of DNA bound to regulatory proteins like the lactose repressor. We report two major findings: First, any non-uniform stiffness tends to lower the energetic cost of looping. Second, the deformation tends to localize in ‘softer’ regions which in turn affects the loop topology as characterized by twist and writhe. The model also offers the capability to calibrate and benchmark experimental measurements of sequence-dependent stiffness. 1

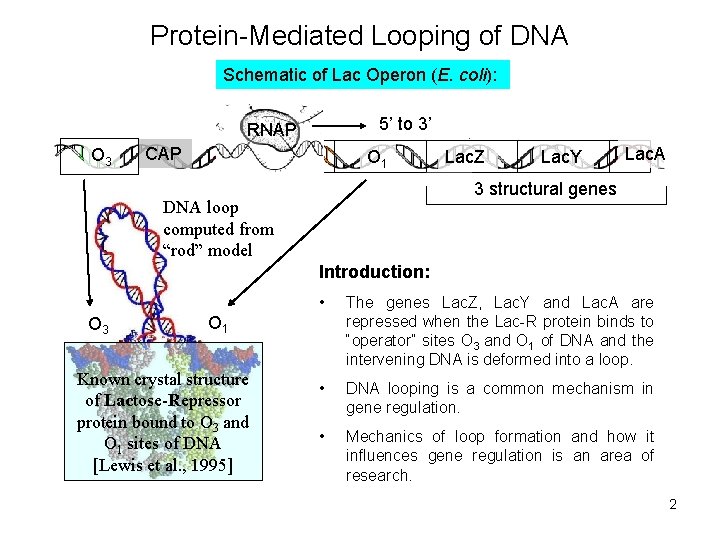
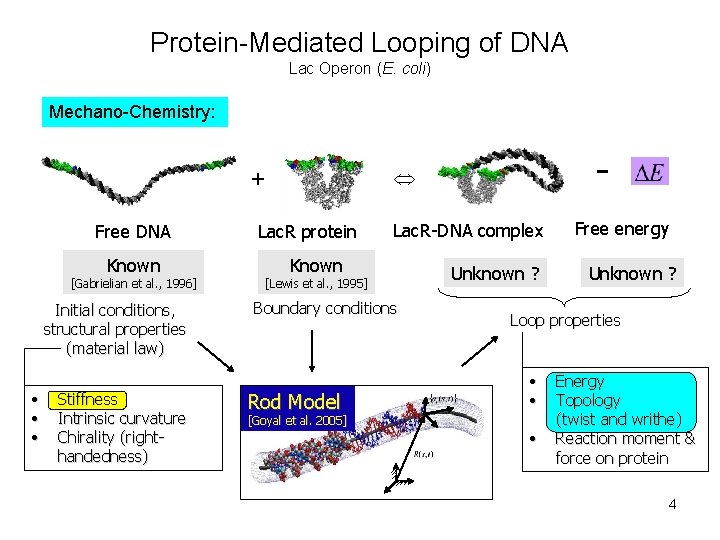
Protein-Mediated Looping of DNA Schematic of Lac Operon (E. coli): 5’ to 3’ RNAP O 3 CAP O 1 Lac. Z Lac. Y Lac. A 3 structural genes DNA loop computed from “rod” model Introduction: O 3 • The genes Lac. Z, Lac. Y and Lac. A are repressed when the Lac-R protein binds to “operator” sites O 3 and O 1 of DNA and the intervening DNA is deformed into a loop. • DNA looping is a common mechanism in gene regulation. • Mechanics of loop formation and how it influences gene regulation is an area of research. O 1 Known crystal structure of Lactose-Repressor protein bound to O 3 and O 1 sites of DNA [Lewis et al. , 1995] 2

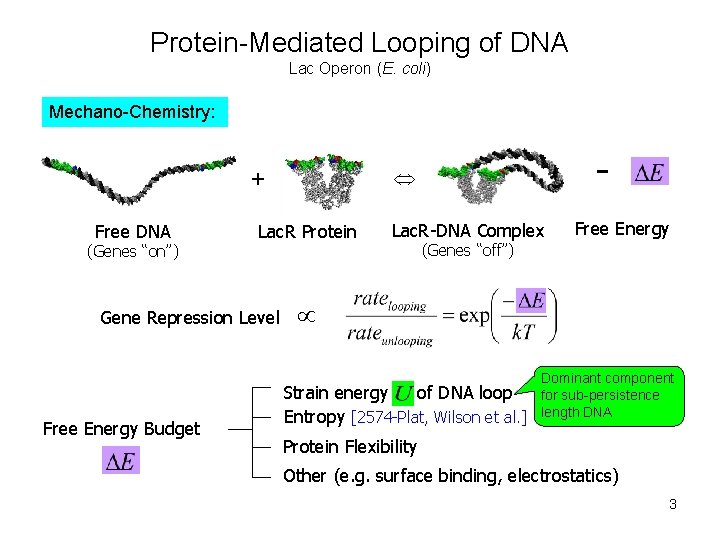
Protein-Mediated Looping of DNA Lac Operon (E. coli) Mechano-Chemistry: + Free DNA (Genes “on”) Lac. R Protein Gene Repression Level Free Energy Budget - Lac. R-DNA Complex Free Energy (Genes “off”) Dominant component Strain energy of DNA loop for sub-persistence Entropy [2574 -Plat, Wilson et al. ] length DNA Protein Flexibility Other (e. g. surface binding, electrostatics) 3

Protein-Mediated Looping of DNA Lac Operon (E. coli) Mechano-Chemistry: + Free DNA Known [Gabrielian et al. , 1996] Initial conditions, structural properties (material law) • • • Stiffness Intrinsic curvature Chirality (righthandedness) - Lac. R protein Lac. R-DNA complex Known [Lewis et al. , 1995] Boundary conditions Rod Model Unknown ? Free energy Unknown ? Loop properties • • [Goyal et al. 2005] • Energy Topology (twist and writhe) Reaction moment & force on protein 4

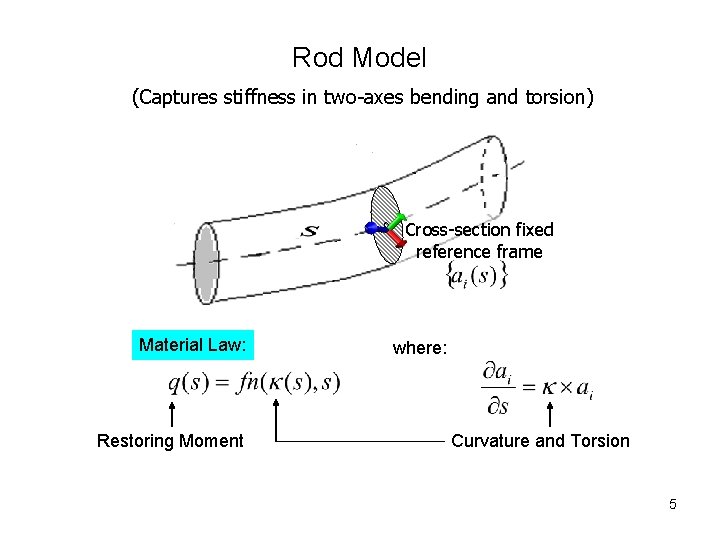
Rod Model (Captures stiffness in two-axes bending and torsion) Cross-section fixed reference frame Material Law: Restoring Moment where: Curvature and Torsion 5
![Formulation of Nonlinear Rod Dynamics Goyal et al 2005 Field Variables v ω Formulation of Nonlinear Rod Dynamics [Goyal et al. , 2005] Field Variables: {v, ω,](https://slidetodoc.com/presentation_image_h2/04646844dfb517ea2ec6e5f5ae5c750e/image-6.jpg)
Formulation of Nonlinear Rod Dynamics [Goyal et al. , 2005] Field Variables: {v, ω, f, κ} (internal force) (internal moment) (velocity) (angular velocity) Field Equations: (curvature & twist) Linear Momentum Equation: Angular Momentum Equation: Free Body Diagram: Inextensibility & Unshearability Constraint: Compatibility Condition: 6

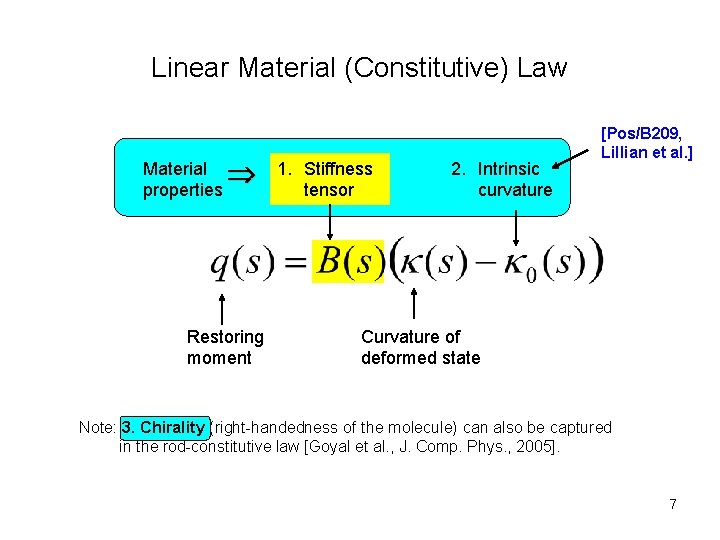
Linear Material (Constitutive) Law Material properties Restoring moment 1. Stiffness tensor 2. Intrinsic curvature [Pos/B 209, Lillian et al. ] Curvature of deformed state Note: 3. Chirality (right-handedness of the molecule) can also be captured in the rod-constitutive law [Goyal et al. , J. Comp. Phys. , 2005]. 7

Linear Material (Constitutive) Law • The material properties are sequence-dependent and hence are non-uniform along the rod-length. • The stiffness tensor includes two-axes bending and torsional stiffness. • Bending stiffness is effectively isotropic on long length-scales due to high intrinsic twist of the molecule [Maddocks and co-workers]. Question: How does the non-uniform stiffness affect DNA looping? 8

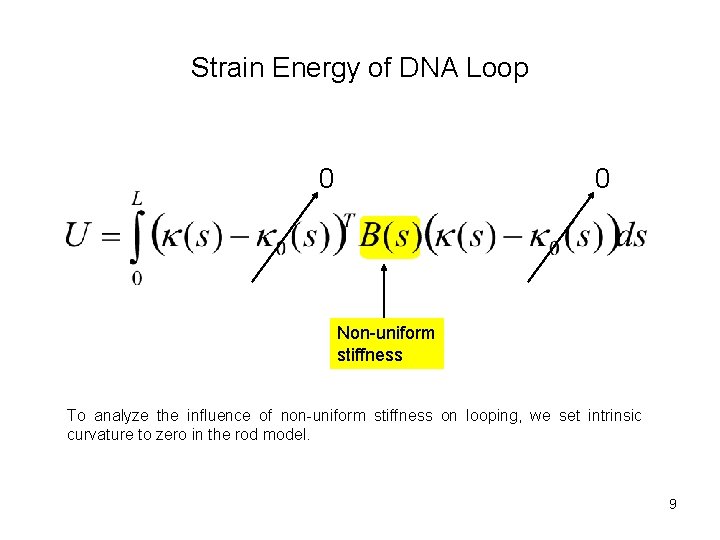
Strain Energy of DNA Loop 0 0 Non-uniform stiffness To analyze the influence of non-uniform stiffness on looping, we set intrinsic curvature to zero in the rod model. 9

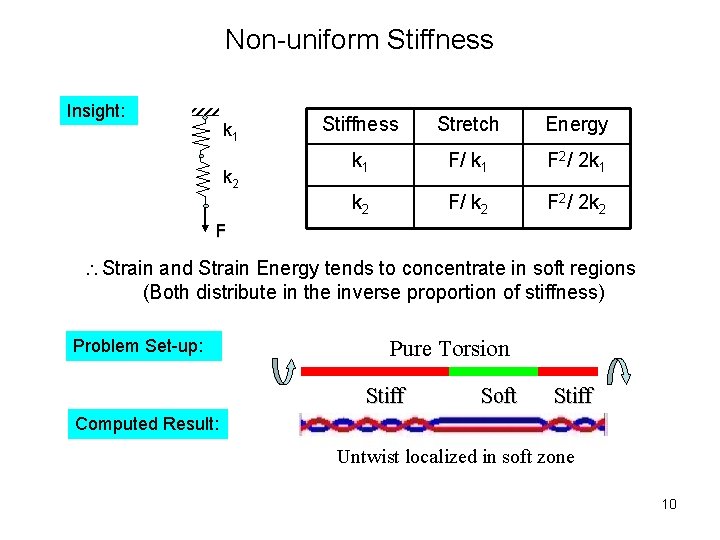
Non-uniform Stiffness Insight: k 1 k 2 Stiffness Stretch Energy k 1 F/ k 1 F 2/ 2 k 1 k 2 F/ k 2 F 2/ 2 k 2 F Strain and Strain Energy tends to concentrate in soft regions (Both distribute in the inverse proportion of stiffness) Problem Set-up: Pure Torsion Stiff Soft Stiff Computed Result: Untwist localized in soft zone 10

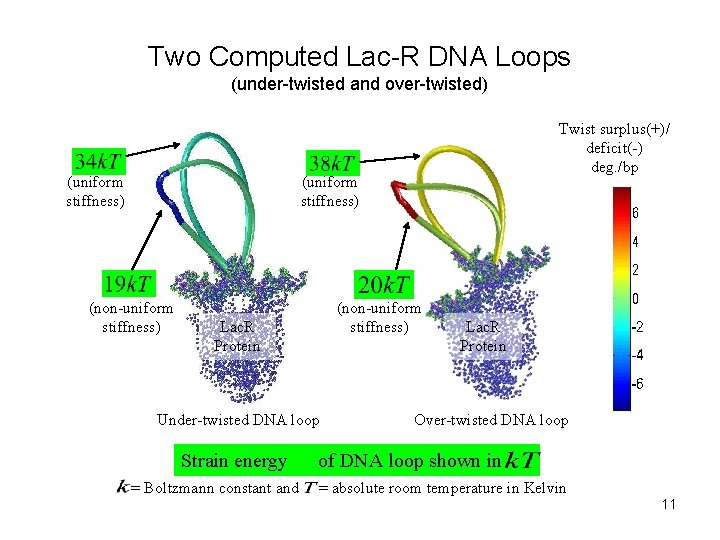
Two Computed Lac-R DNA Loops (under-twisted and over-twisted) (uniform stiffness) Twist surplus(+)/ deficit(-) deg. /bp (uniform stiffness) (non-uniform stiffness) Lac. R Protein Under-twisted DNA loop Strain energy = Boltzmann constant and Lac. R Protein Over-twisted DNA loop of DNA loop shown in = absolute room temperature in Kelvin 11

Figure Description The figure shows a simulation example pertaining to Lac. R-DNA loops where the stiffness is lowered by an order of magnitude at a specified location (see next slide). The results are contrasted with those predicted by the rod with uniform stiffness. The color scale shows the distribution of twist surplus (+) or deficit (-) over the nominal twist of 34. 6° per basepair step. Observations • Twist/ untwist and bending localizes in the softer region. • Strain energy of the loop is lowered with non-uniform stiffness. 12

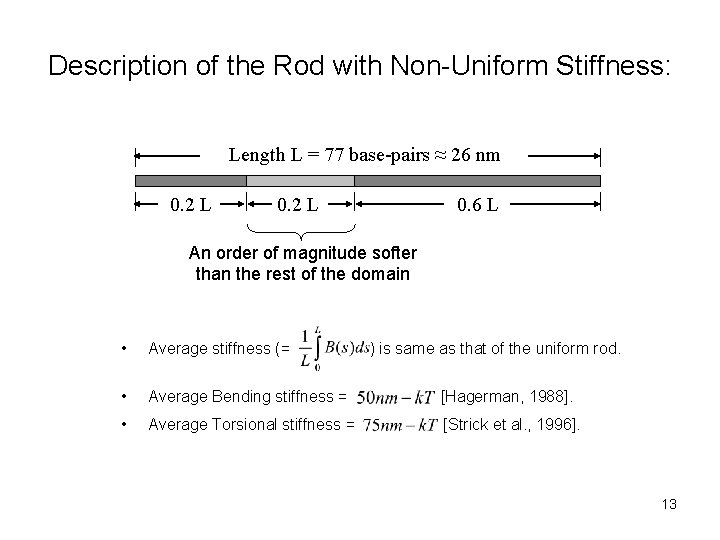
Description of the Rod with Non-Uniform Stiffness: Length L = 77 base-pairs ≈ 26 nm 0. 2 L 0. 6 L An order of magnitude softer than the rest of the domain • Average stiffness (= • Average Bending stiffness = [Hagerman, 1988]. • Average Torsional stiffness = [Strick et al. , 1996]. ) is same as that of the uniform rod. 13

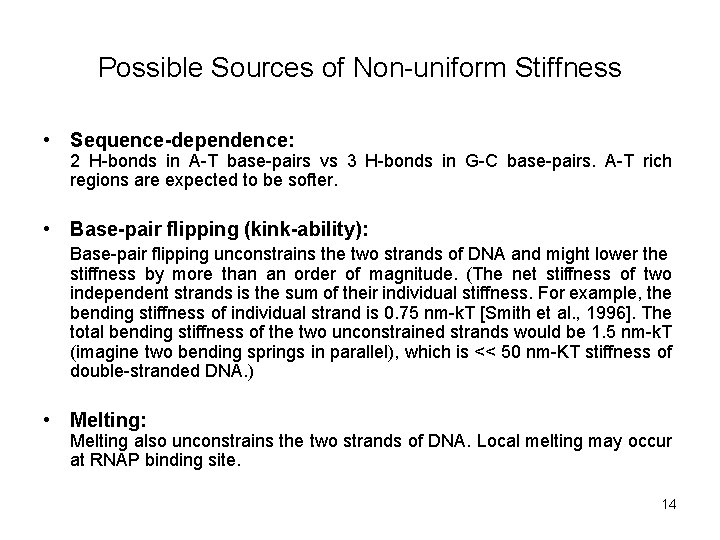
Possible Sources of Non-uniform Stiffness • Sequence-dependence: 2 H-bonds in A-T base-pairs vs 3 H-bonds in G-C base-pairs. A-T rich regions are expected to be softer. • Base-pair flipping (kink-ability): Base-pair flipping unconstrains the two strands of DNA and might lower the stiffness by more than an order of magnitude. (The net stiffness of two independent strands is the sum of their individual stiffness. For example, the bending stiffness of individual strand is 0. 75 nm-k. T [Smith et al. , 1996]. The total bending stiffness of the two unconstrained strands would be 1. 5 nm-k. T (imagine two bending springs in parallel), which is << 50 nm-KT stiffness of double-stranded DNA. ) • Melting: Melting also unconstrains the two strands of DNA. Local melting may occur at RNAP binding site. 14

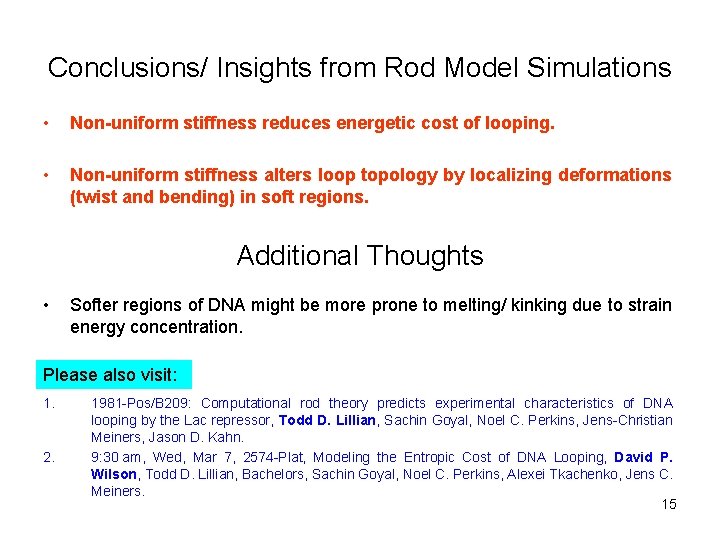
Conclusions/ Insights from Rod Model Simulations • Non-uniform stiffness reduces energetic cost of looping. • Non-uniform stiffness alters loop topology by localizing deformations (twist and bending) in soft regions. Additional Thoughts • Softer regions of DNA might be more prone to melting/ kinking due to strain energy concentration. Please also visit: 1. 2. 1981 -Pos/B 209: Computational rod theory predicts experimental characteristics of DNA looping by the Lac repressor, Todd D. Lillian, Sachin Goyal, Noel C. Perkins, Jens-Christian Meiners, Jason D. Kahn. 9: 30 am, Wed, Mar 7, 2574 -Plat, Modeling the Entropic Cost of DNA Looping, David P. Wilson, Todd D. Lillian, Bachelors, Sachin Goyal, Noel C. Perkins, Alexei Tkachenko, Jens C. Meiners. 15

Online Reference Acknowledgements: (NSF, ONR, LLNL) Website contents: (handout (PPT), publications) Special thanks to: (Andricioaei et al, Tkachenko et al. ) Questions/ comments e. mail to: http: //www. whoi. edu/sites/sgoyal (Go paperless, go blue!) Sachin Goyal sgoyal@umich. edu Todd Lillian tlillian@umich. edu David Wilson doros@umich. edu Edgar Meyhofer meyhofer@umich. edu Jens-Christian Meiners meiners@umich. edu Noel Perkins ncp@umich. edu 16
 Hemoptasis
Hemoptasis Mitesh kapra
Mitesh kapra Sachin bhatkar
Sachin bhatkar Sachin adlakha
Sachin adlakha Chd pulmonary hypertension
Chd pulmonary hypertension Sachin amrute
Sachin amrute Sachin thorbole
Sachin thorbole Sachin talathi
Sachin talathi Sachin kheterpal
Sachin kheterpal Popliteal artery
Popliteal artery Sachin kale eye specialist
Sachin kale eye specialist Virtualization wireless infrastructure
Virtualization wireless infrastructure Dr mahesh marda
Dr mahesh marda Function of dna polymerase 3
Function of dna polymerase 3 Coding dna and non coding dna
Coding dna and non coding dna Bioflix activity dna replication dna replication diagram
Bioflix activity dna replication dna replication diagram Replication process
Replication process