Sensitivity and specificity of Clostridium difficile detection kits











- Slides: 26

Sensitivity and specificity of Clostridium difficile detection kits Kerrie Eastwood Clinical Scientist Leeds Teaching Hospitals NHS Trust

Overview § § § § Background on C. difficile Purpose of study Methods Results Which kit is best? What’s next? Acknowledgements

Background • Anerobic spore-forming bacilli – survive in environment – Need to wash hands • Nosocomial pathogen – Predisposing antibiotics • Cephalosporins • Clindamycin • Fluoroquinolones • Cross infection

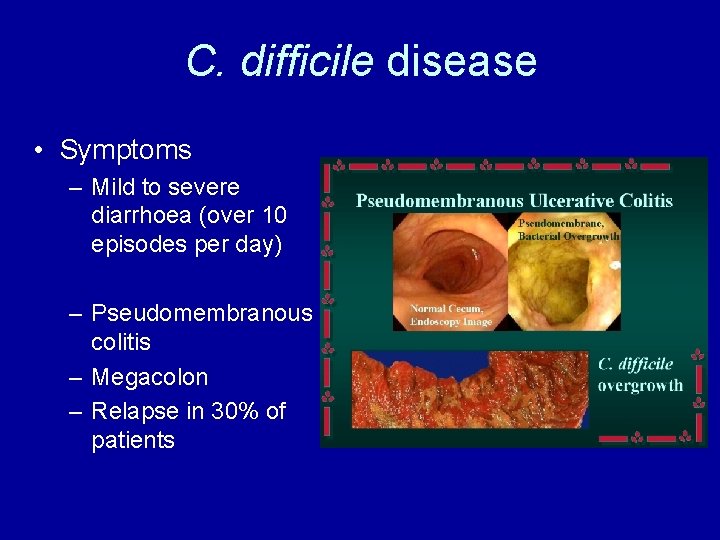
C. difficile disease • Symptoms – Mild to severe diarrhoea (over 10 episodes per day) – Pseudomembranous colitis – Megacolon – Relapse in 30% of patients

Diagnosis and treatment • Laboratory diagnosis – Don’t just isolate organism – Detection of toxin • Treatment – Stop predisposing antibiotics – Start oral metronidazole (or Vancomycin if severe or ribotype 027) – Infection control e. g. isolation/cohorting

Purpose of study • No real comparison to date • Evidence based on small studies – Debunked by manufacturers • False positives?

Implications of false positive CDI diagnosis • Inappropriate antibiotic cessation / modification • Inappropriate treatment for CDI • Unnecessary isolation • Potentially harmful cohorting • Inaccurate surveillance / infection control data • Wasted resources • Reimbursement / fines • Medicolegal implications

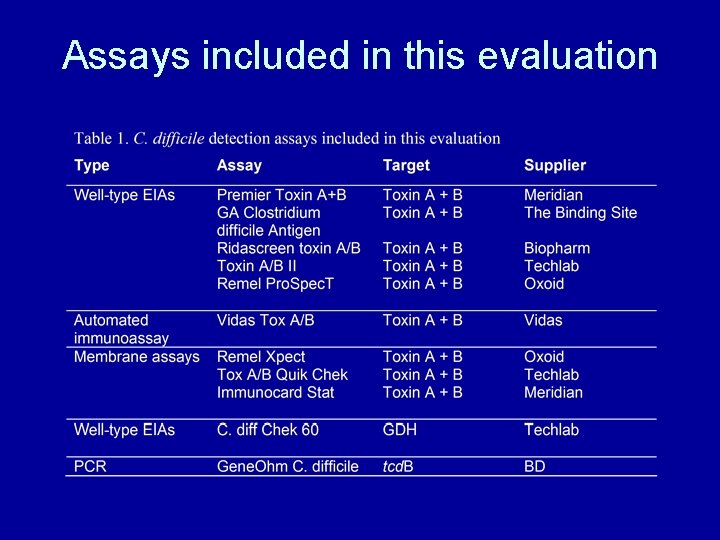
Types of commercial toxin detection assay Enzyme immunoassay • 96 -well format • manual • Semi-automated Enzyme-linked Fluorescence assay • Automated Lateral flow assay • Rapid

Other commercial tests for C. difficile • Glutamate dehydrogenase (GDH) – Cell surface associated enzyme – Found in many bacterial species – EIA assay specific for C. difficile GDH • Real time PCR – Detection of toxin B gene – Doesn’t indicate toxin production – Alternative assays available to detect other toxin genes

Assays included in this evaluation

Gold standards • Two gold standards used for comparison – Cytotoxin assay – Cytotoxigenic culture • Cytotoxin assay performed on culture supernatants

Sample selection • Collected 600 samples – Submitted for C. difficile testing – Diarrhoeal – Enough volume • Picked daily (10 per day) • Randomised anonymised before testing • PCR (n=554) and GDH (n=558) performed on freeze-thawed samples at later date

Sample processing • Each sample – – – tested on every assay Cultured on CCEYL agar in anaerobic cabinet Cytotoxin Cytotoxigenic culture Isolates stored at -70°C Isolates PCR-ribotype • Discordant results for toxin detection assays – Majority rules – Repeated further 2 times (best of 3)

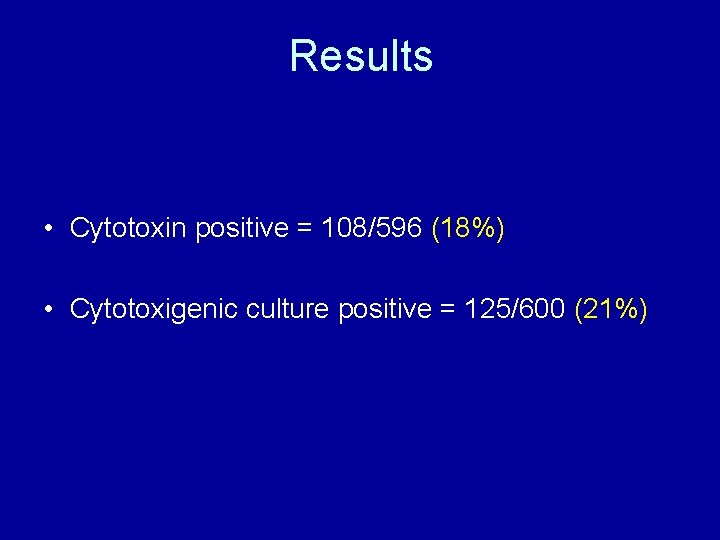
Results • Cytotoxin positive = 108/596 (18%) • Cytotoxigenic culture positive = 125/600 (21%)

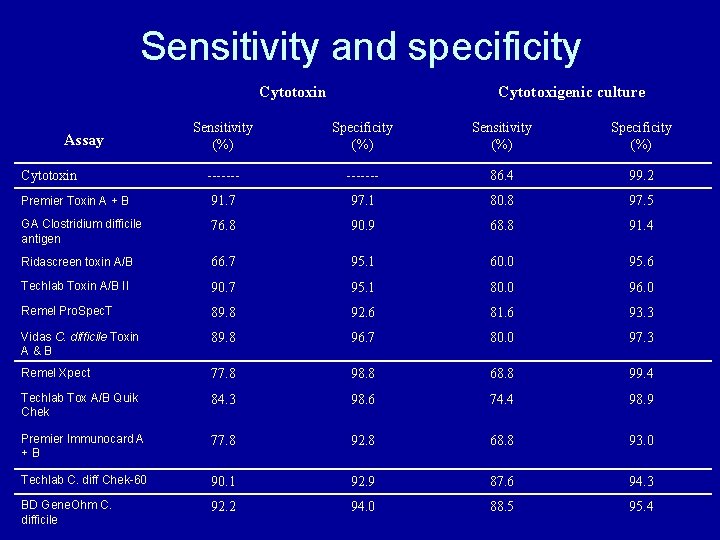
Sensitivity and specificity Cytotoxin Cytotoxigenic culture Sensitivity (%) Specificity (%) ------- 86. 4 99. 2 Premier Toxin A + B 91. 7 97. 1 80. 8 97. 5 GA Clostridium difficile antigen 76. 8 90. 9 68. 8 91. 4 Ridascreen toxin A/B 66. 7 95. 1 60. 0 95. 6 Techlab Toxin A/B II 90. 7 95. 1 80. 0 96. 0 Remel Pro. Spec. T 89. 8 92. 6 81. 6 93. 3 Vidas C. difficile Toxin A&B 89. 8 96. 7 80. 0 97. 3 Remel Xpect 77. 8 98. 8 68. 8 99. 4 Techlab Tox A/B Quik Chek 84. 3 98. 6 74. 4 98. 9 Premier Immunocard A +B 77. 8 92. 8 68. 8 93. 0 Techlab C. diff Chek-60 90. 1 92. 9 87. 6 94. 3 BD Gene. Ohm C. difficile 92. 2 94. 0 88. 5 95. 4 Assay Cytotoxin

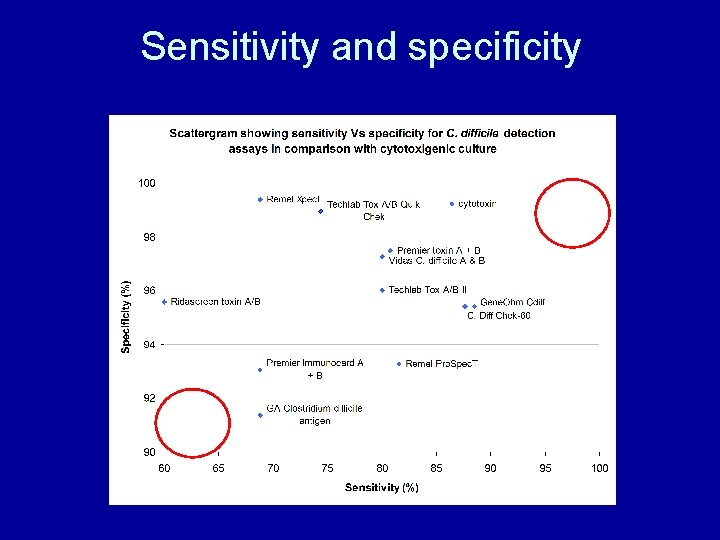
Sensitivity and specificity

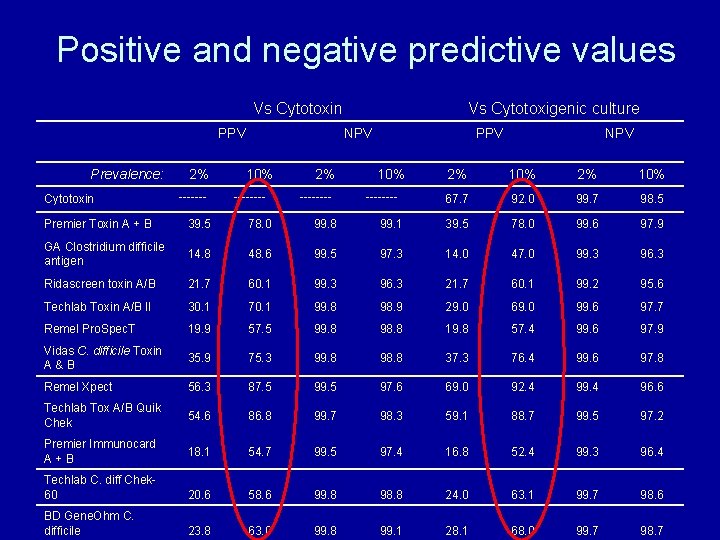
Positive and negative predictive values Change depending on the prevalence of toxin positive C. difficile in faecal samples within the population • 10% prevalence in hospital setting • 2% prevalence in community setting

Positive and negative predictive values Vs Cytotoxin PPV Prevalence: Cytotoxin 2% ------- Vs Cytotoxigenic culture NPV 10% ---- 2% ---- PPV 10% ---- NPV 2% 10% 67. 7 92. 0 99. 7 98. 5 Premier Toxin A + B 39. 5 78. 0 99. 8 99. 1 39. 5 78. 0 99. 6 97. 9 GA Clostridium difficile antigen 14. 8 48. 6 99. 5 97. 3 14. 0 47. 0 99. 3 96. 3 Ridascreen toxin A/B 21. 7 60. 1 99. 3 96. 3 21. 7 60. 1 99. 2 95. 6 Techlab Toxin A/B II 30. 1 70. 1 99. 8 98. 9 29. 0 69. 0 99. 6 97. 7 Remel Pro. Spec. T 19. 9 57. 5 99. 8 98. 8 19. 8 57. 4 99. 6 97. 9 Vidas C. difficile Toxin A&B 35. 9 75. 3 99. 8 98. 8 37. 3 76. 4 99. 6 97. 8 Remel Xpect 56. 3 87. 5 99. 5 97. 6 69. 0 92. 4 99. 4 96. 6 Techlab Tox A/B Quik Chek 54. 6 86. 8 99. 7 98. 3 59. 1 88. 7 99. 5 97. 2 Premier Immunocard A+B 18. 1 54. 7 99. 5 97. 4 16. 8 52. 4 99. 3 96. 4 Techlab C. diff Chek 60 20. 6 58. 6 99. 8 98. 8 24. 0 63. 1 99. 7 98. 6 BD Gene. Ohm C. difficile 23. 8 63. 0 99. 8 99. 1 28. 1 68. 0 99. 7 98. 7

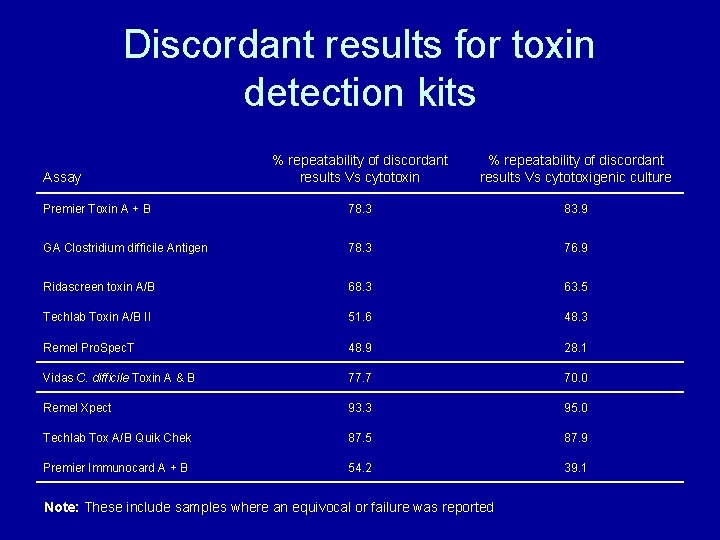
Discordant results for toxin detection kits % repeatability of discordant results Vs cytotoxin % repeatability of discordant results Vs cytotoxigenic culture Premier Toxin A + B 78. 3 83. 9 GA Clostridium difficile Antigen 78. 3 76. 9 Ridascreen toxin A/B 68. 3 63. 5 Techlab Toxin A/B II 51. 6 48. 3 Remel Pro. Spec. T 48. 9 28. 1 Vidas C. difficile Toxin A & B 77. 7 70. 0 Remel Xpect 93. 3 95. 0 Techlab Tox A/B Quik Chek 87. 5 87. 9 Premier Immunocard A + B 54. 2 39. 1 Assay Note: These include samples where an equivocal or failure was reported

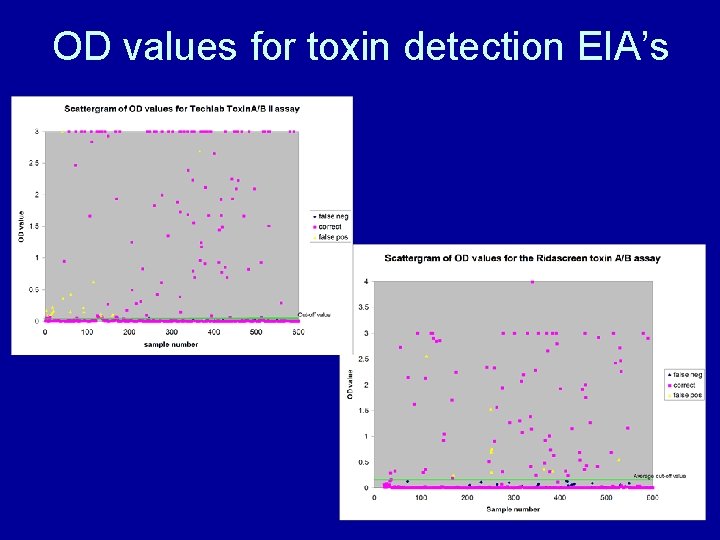
OD values for toxin detection EIA’s

Ribotypes • 128 culture positive samples, of which 125 were cytotoxin positive • There were 21 different ribotypes; most common ribotypes – 106 (26. 6%) – 027 (18. 8%) – 002 (6. 3%) • No difference between assays for different ribotypes

Which kit is best? • Depends on your population • Cytotoxin gives best PPV for toxin detection assays – But is labour intensive and slow • Lateral flow toxin detection assays have good PPV and are rapid – But have poorer NPV • GDH gives best PPV overall – But is only detecting presence of C. difficile, not active disease • PCR has highest NPV, good screening test – But only detecting presence of toxin gene • Test results should be taken in context with the clinical presentation of the patient

Single tests? Advice from the Department of Health: • The currently available kits for detection of C. difficile toxins have variable performance • Currently available kits may miss about 1 in 5 to 1 in 10 cases of CDI and will falsely identify (1 -2 out of 10) cases as positive when they are not • The poor positive predictive values of toxin detection kits, especially in the context of widespread testing, and the possibility of missing true positives mean that there are limitations to using these as single tests for the laboratory diagnosis of CDI

What’s next? • Algorithms – Two step – Three step – Which combination of tests? • Requires further evaluation

Acknowledgements • • • Prof. Mark Wilcox Patrick Else All the Enteric lab staff All the manufacturers/distributors Ann Prothero (Leeds Ethics) Keith Perry and Andre Charlett at HPA

Any questions? Useful references: ØComparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcd. B, and a glutamate dyhydrogenase assay to cytotoxin testing and cytotoxigenic culture methods. 2009. Eastwood K. , Else P. , Charlett A. and Wilcox M. Journal of Clinical Microbiology. 47: 32113217 Øhttp: //www. pasa. nhs. uk/pasa/Doc. aspx? Path=%5 b. MN%5 d%5 b. SP%5 d/NHSprocurement/CEP 08054. p df CEP report on toxin detection methods. Øhttp: //www. hpa. org. uk/hpr/archives/2009/news 1209. htm#cdtdks DOH advice on usingle tests.