Seminar on INTRODUCTION TO SOLID STATE PHYSICS Presented





- Slides: 9

Seminar on INTRODUCTION TO SOLID STATE PHYSICS Presented By D. M. Parshuramkar Dept. Of physics, N. H. College, bramhapuri


What is Solid State Physics? n Explains the properties of solid materials. n Demonstrate Crystallography of solids. n Explains the properties of a collection of atomic nuclei and electrons interacting with electrostatic forces. n Formulates fundamental laws that govern the behaviour of solids.

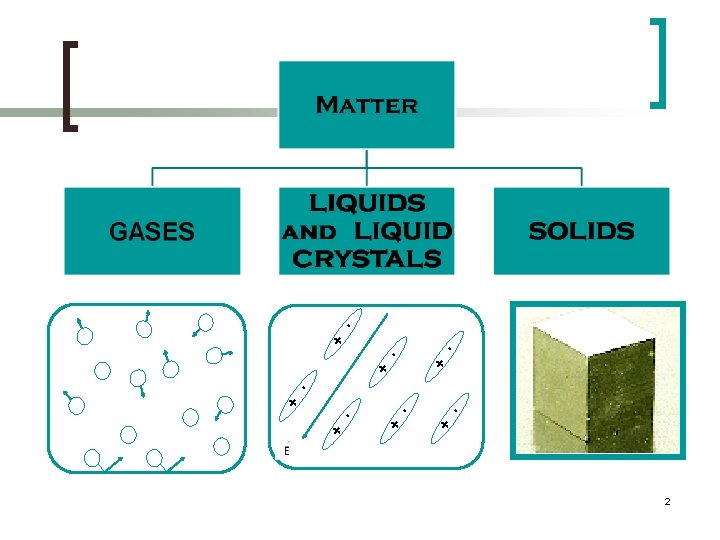
CLASSIFICATION OF SOLIDS SOLID MATERIALS CRYSTALLINE POLYCRYSTALLINE AMORPHOUS (Non-crystalline) Single Crystal Structure 4

Crystalline Solids n Crystalline materials are solids with an atomic structure based on a regular repeated pattern. n The majority of all solids are crystalline. n More progress has been made in understanding the behaviour of crystalline solids than that of noncrystalline materials since crystalline materials are more stable. n Examples includes Most Metals, Ceramics etc.

Amorphous Solids n Amorphous Solids are made up of randomly orientated atoms, ions, or molecules that do not form defined patterns or lattice structures. n Amorphous materials have order only within a few atomic or molecular dimensions. n Amorphous materials do not have any long-range order, but they have varying degrees of short-range order. n Examples include amorphous silicon, plastics, and glasses. 6

Why Most Solids are Crystalline in Nature. . ? Ø Nature always loves the Symmetry. Ø Symmetry measures the Stability. Ø Stability indicates the state with less energy. Ø Crystalline solids exists with less energy. Ø Therefore Crystalline solids are more stable. 7

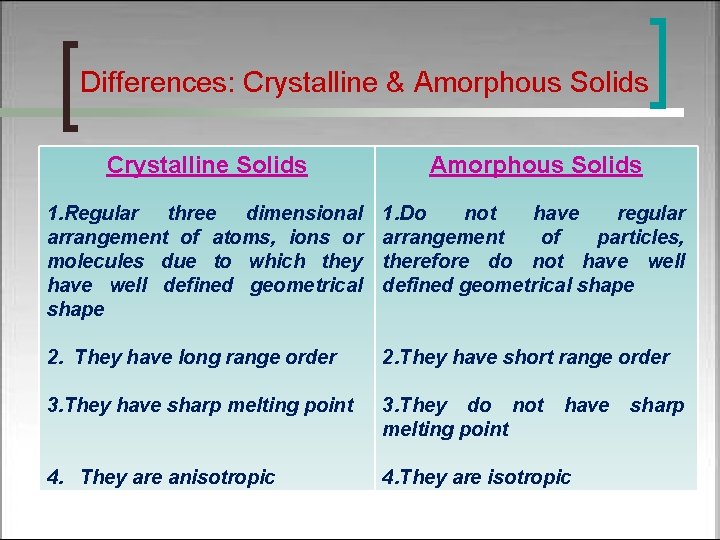
Differences: Crystalline & Amorphous Solids Crystalline Solids Amorphous Solids 1. Regular three dimensional arrangement of atoms, ions or molecules due to which they have well defined geometrical shape 1. Do not have regular arrangement of particles, therefore do not have well defined geometrical shape 2. They have long range order 2. They have short range order 3. They have sharp melting point 3. They do not melting point 4. They are anisotropic 4. They are isotropic have sharp

Thank you 9