Semiconductor Devices Atoms and electricity Semiconductor structure Conduction



- Slides: 23

Semiconductor Devices · · Atoms and electricity Semiconductor structure Conduction in semiconductors Doping – epitaxy – diffusion – ion implantation · Transistors – MOS – CMOS · Implementing logic functions (4. 1)

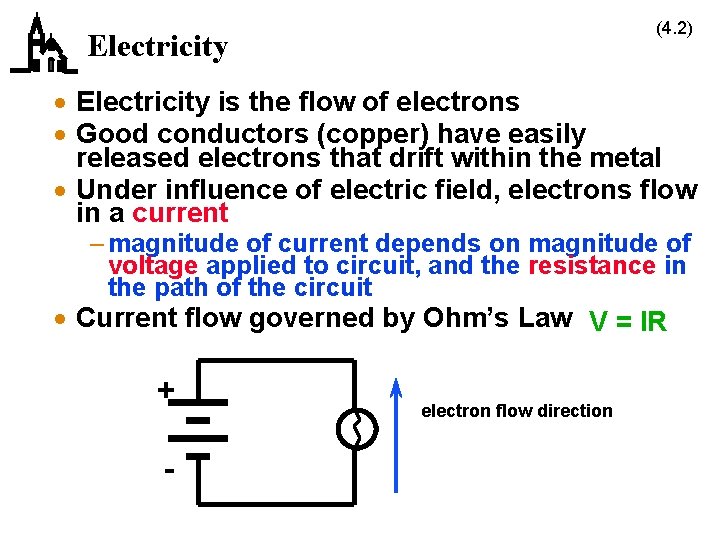
(4. 2) Electricity · Electricity is the flow of electrons · Good conductors (copper) have easily released electrons that drift within the metal · Under influence of electric field, electrons flow in a current – magnitude of current depends on magnitude of voltage applied to circuit, and the resistance in the path of the circuit · Current flow governed by Ohm’s Law V = IR + - electron flow direction

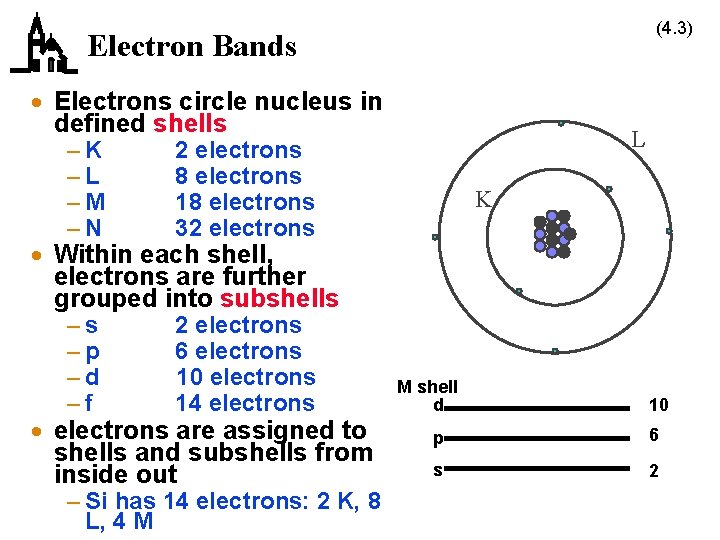
(4. 3) Electron Bands · Electrons circle nucleus in defined shells –K –L –M –N 2 electrons 8 electrons 18 electrons 32 electrons –s –p –d –f 2 electrons 6 electrons 10 electrons 14 electrons L K · Within each shell, electrons are further grouped into subshells · electrons are assigned to shells and subshells from inside out – Si has 14 electrons: 2 K, 8 L, 4 M M shell d 10 p 6 s 2

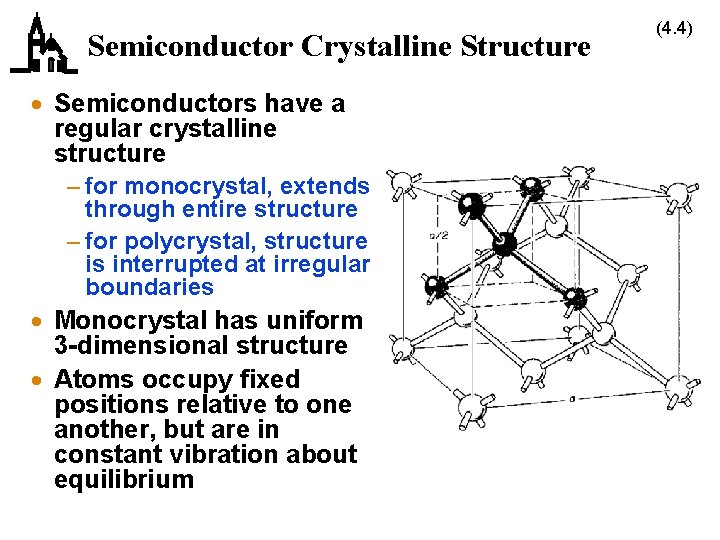
Semiconductor Crystalline Structure · Semiconductors have a regular crystalline structure – for monocrystal, extends through entire structure – for polycrystal, structure is interrupted at irregular boundaries · Monocrystal has uniform 3 -dimensional structure · Atoms occupy fixed positions relative to one another, but are in constant vibration about equilibrium (4. 4)

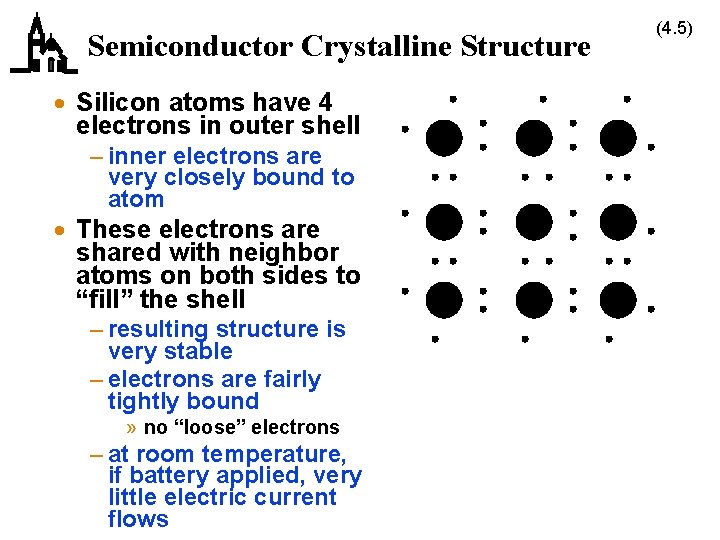
Semiconductor Crystalline Structure · Silicon atoms have 4 electrons in outer shell – inner electrons are very closely bound to atom · These electrons are shared with neighbor atoms on both sides to “fill” the shell – resulting structure is very stable – electrons are fairly tightly bound » no “loose” electrons – at room temperature, if battery applied, very little electric current flows (4. 5)

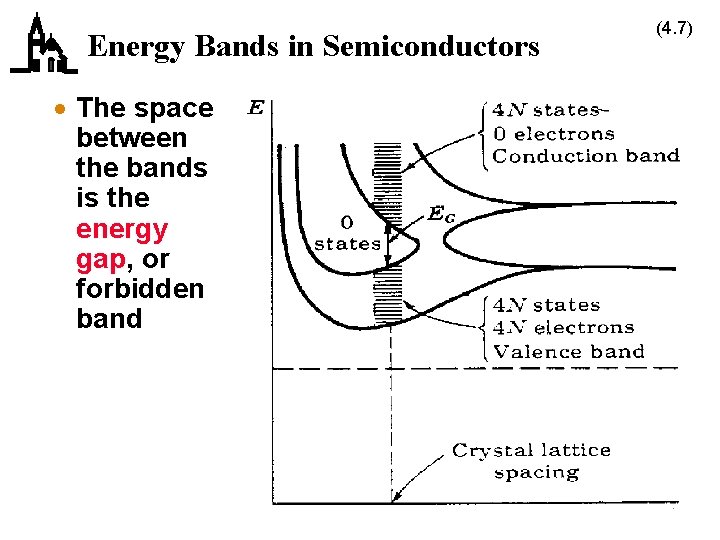
Conduction in Crystal Lattices (4. 6) · Semiconductors (Si and Ge) have 4 electrons in their outer shell – 2 in the s subshell – 2 in the p subshell · As the distance between atoms decreases the discrete subshells spread out into bands · As the distance decreases further, the bands overlap and then separate – the subshell model doesn’t hold anymore, and the electrons can be thought of as being part of the crystal, not part of the atom – 4 possible electrons in the lower band (valence band) – 4 possible electrons in the upper band (conduction band)

Energy Bands in Semiconductors · The space between the bands is the energy gap, or forbidden band (4. 7)

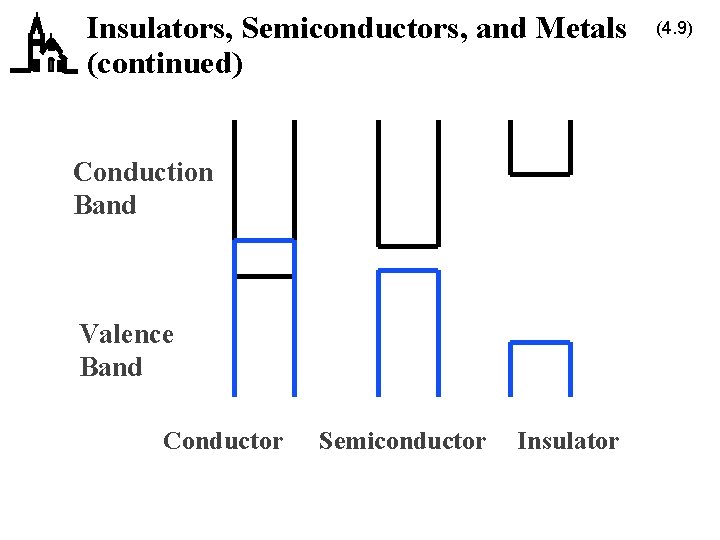
Insulators, Semiconductors, and Metals (4. 8) · This separation of the valence and conduction bands determines the electrical properties of the material · Insulators have a large energy gap – electrons can’t jump from valence to conduction bands – no current flows · Conductors (metals) have a very small (or nonexistent) energy gap – electrons easily jump to conduction bands due to thermal excitation – current flows easily · Semiconductors have a moderate energy gap – only a few electrons can jump to the conduction band » leaving “holes” – only a little current can flow

Insulators, Semiconductors, and Metals (continued) Conduction Band Valence Band Conductor Semiconductor Insulator (4. 9)

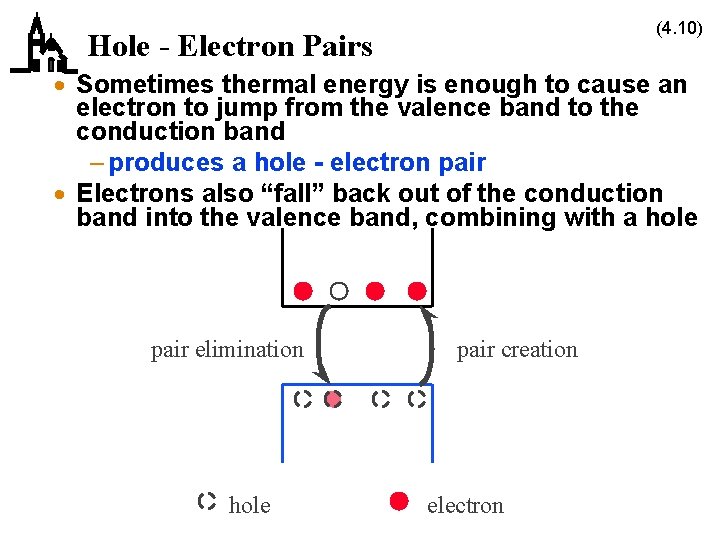
(4. 10) Hole - Electron Pairs · Sometimes thermal energy is enough to cause an electron to jump from the valence band to the conduction band – produces a hole - electron pair · Electrons also “fall” back out of the conduction band into the valence band, combining with a hole pair elimination hole pair creation electron

Improving Conduction by Doping (4. 11) · To make semiconductors better conductors, add impurities (dopants) to contribute extra electrons or extra holes – elements with 5 outer electrons contribute an extra electron to the lattice (donor dopant) – elements with 3 outer electrons accept an electron from the silicon (acceptor dopant)

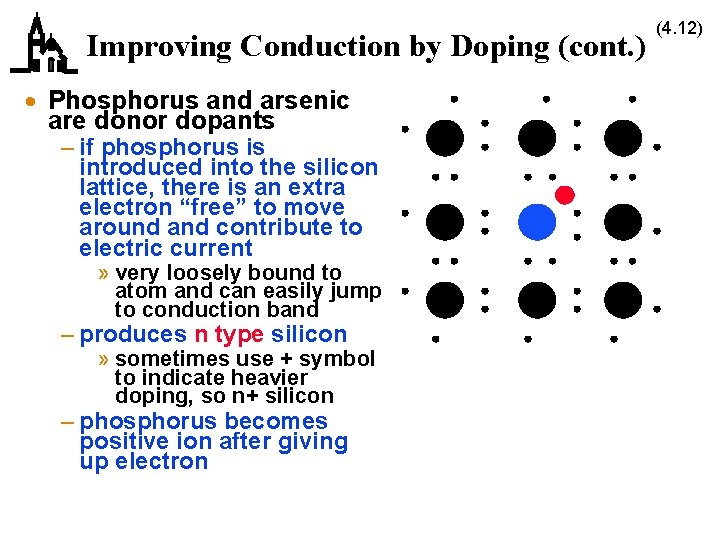
Improving Conduction by Doping (cont. ) · Phosphorus and arsenic are donor dopants – if phosphorus is introduced into the silicon lattice, there is an extra electron “free” to move around and contribute to electric current » very loosely bound to atom and can easily jump to conduction band – produces n type silicon » sometimes use + symbol to indicate heavier doping, so n+ silicon – phosphorus becomes positive ion after giving up electron (4. 12)

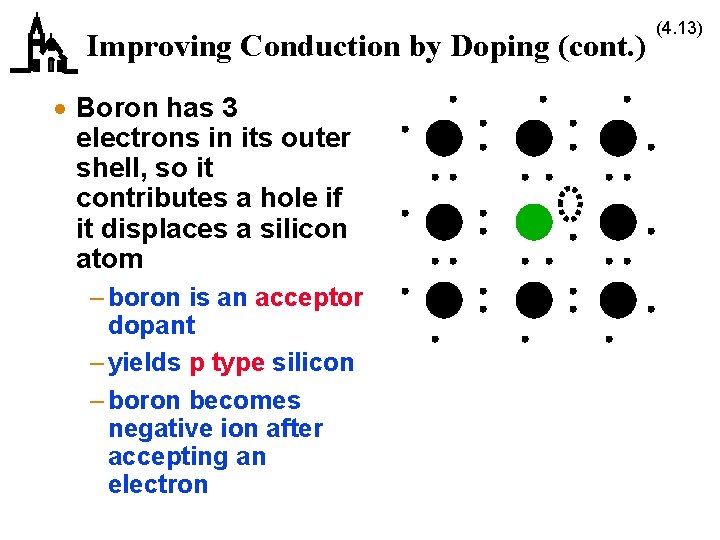
Improving Conduction by Doping (cont. ) · Boron has 3 electrons in its outer shell, so it contributes a hole if it displaces a silicon atom – boron is an acceptor dopant – yields p type silicon – boron becomes negative ion after accepting an electron (4. 13)

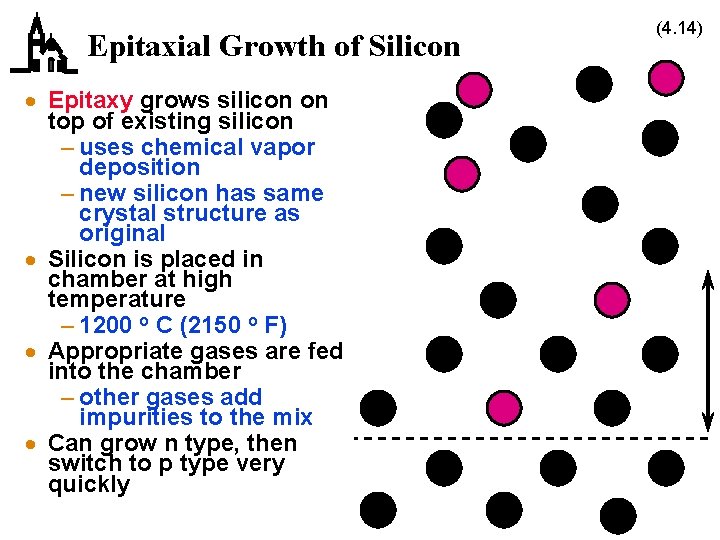
Epitaxial Growth of Silicon · Epitaxy grows silicon on top of existing silicon – uses chemical vapor deposition – new silicon has same crystal structure as original · Silicon is placed in chamber at high temperature – 1200 o C (2150 o F) · Appropriate gases are fed into the chamber – other gases add impurities to the mix · Can grow n type, then switch to p type very quickly (4. 14)

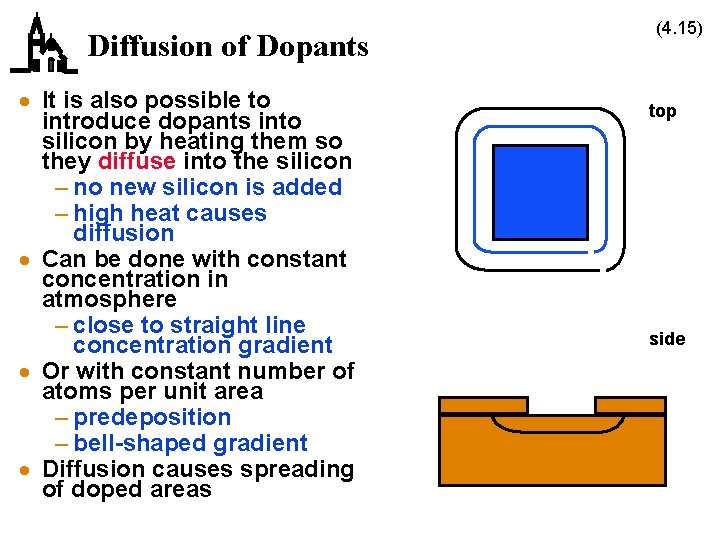
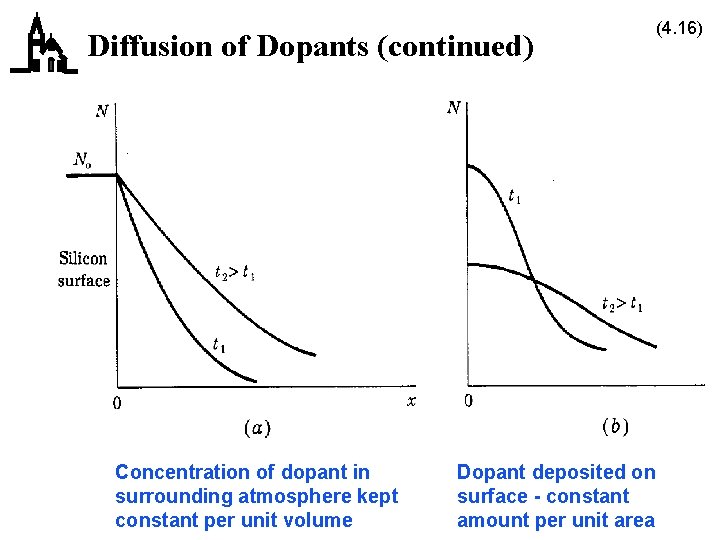
Diffusion of Dopants · It is also possible to introduce dopants into silicon by heating them so they diffuse into the silicon – no new silicon is added – high heat causes diffusion · Can be done with constant concentration in atmosphere – close to straight line concentration gradient · Or with constant number of atoms per unit area – predeposition – bell-shaped gradient · Diffusion causes spreading of doped areas (4. 15) top side

Diffusion of Dopants (continued) Concentration of dopant in surrounding atmosphere kept constant per unit volume Dopant deposited on surface - constant amount per unit area (4. 16)

Ion Implantation of Dopants (4. 17) · One way to reduce the spreading found with diffusion is to use ion implantation – also gives better uniformity of dopant – yields faster devices – lower temperature process · Ions are accelerated from 5 Kev to 10 Mev and directed at silicon – higher energy gives greater depth penetration – total dose is measured by flux » number of ions per cm 2 » typically 1012 per cm 2 - 1016 per cm 2 · Flux is over entire surface of silicon – use masks to cover areas where implantation is not wanted · Heat afterward to work into crystal lattice

Hole and Electron Concentrations (4. 18) · To produce reasonable levels of conduction doesn’t require much doping – silicon has about 5 x 1022 atoms/cm 3 – typical dopant levels are about 1015 atoms/cm 3 · In undoped (intrinsic) silicon, the number of holes and number of free electrons is equal, and their product equals a constant – actually, ni increases with increasing temperature np = ni 2 · This equation holds true for doped silicon as well, so increasing the number of free electrons decreases the number of holes

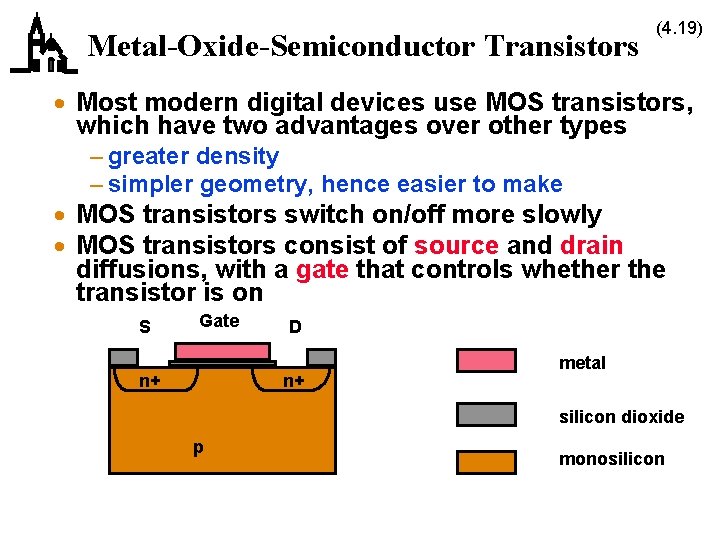
Metal-Oxide-Semiconductor Transistors (4. 19) · Most modern digital devices use MOS transistors, which have two advantages over other types – greater density – simpler geometry, hence easier to make · MOS transistors switch on/off more slowly · MOS transistors consist of source and drain diffusions, with a gate that controls whether the transistor is on S Gate n+ D n+ metal silicon dioxide p monosilicon

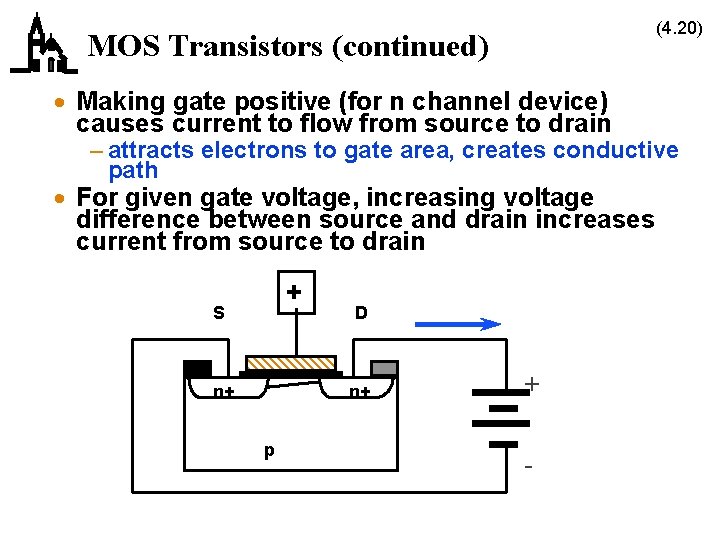
(4. 20) MOS Transistors (continued) · Making gate positive (for n channel device) causes current to flow from source to drain – attracts electrons to gate area, creates conductive path · For given gate voltage, increasing voltage difference between source and drain increases current from source to drain + S n+ D n+ p + -

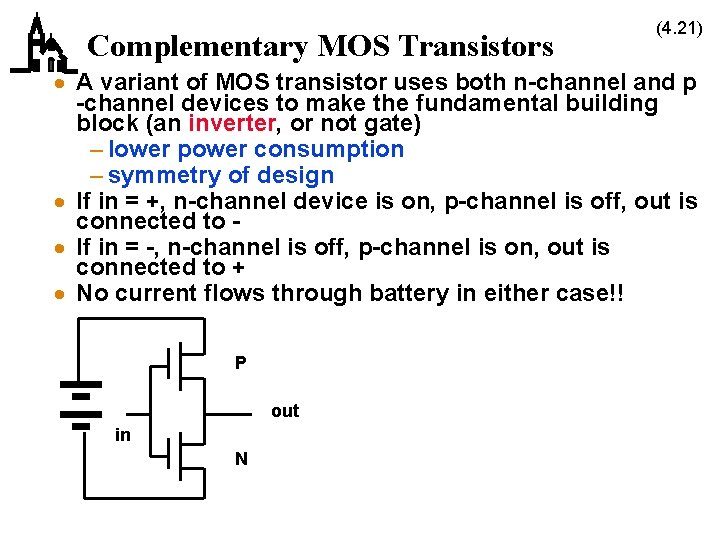
Complementary MOS Transistors (4. 21) · A variant of MOS transistor uses both n-channel and p -channel devices to make the fundamental building block (an inverter, or not gate) – lower power consumption – symmetry of design · If in = +, n-channel device is on, p-channel is off, out is connected to · If in = -, n-channel is off, p-channel is on, out is connected to + · No current flows through battery in either case!! P out in N

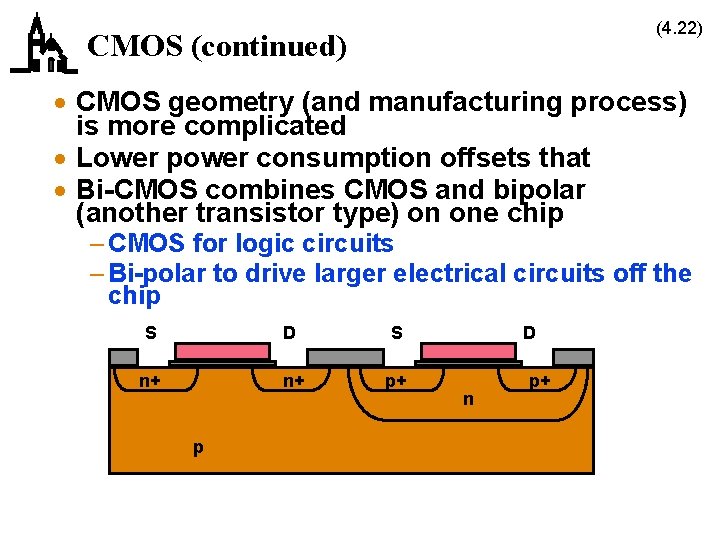
(4. 22) CMOS (continued) · CMOS geometry (and manufacturing process) is more complicated · Lower power consumption offsets that · Bi-CMOS combines CMOS and bipolar (another transistor type) on one chip – CMOS for logic circuits – Bi-polar to drive larger electrical circuits off the chip S D S n+ n+ p+ p D n p+

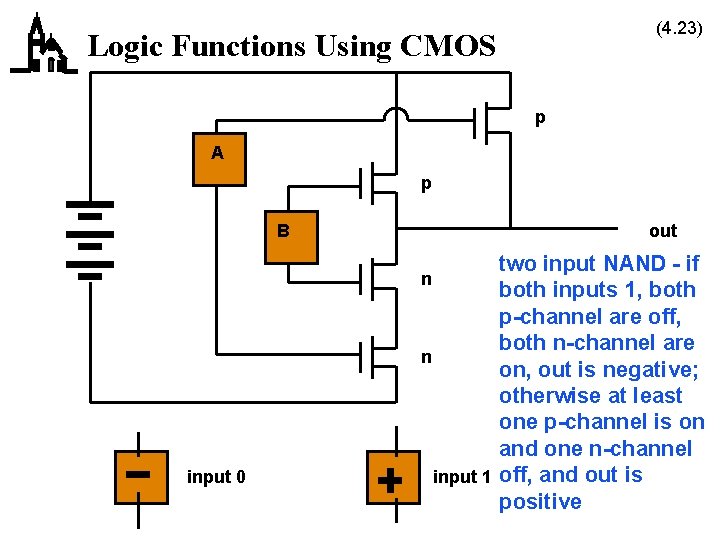
(4. 23) Logic Functions Using CMOS p A p B input 0 out two input NAND - if n both inputs 1, both p-channel are off, both n-channel are n on, out is negative; otherwise at least one p-channel is on and one n-channel input 1 off, and out is positive