Semester Two Final Review 2016 Chemistry Stoichiometry Balanced

Semester Two Final Review 2016 Chemistry

Stoichiometry – Balanced Equations ● A balanced chemical equation tells the ratio of the number of moles of one compound to moles of another compound Another example… 2 Co. Br 3 + 3 Ca. SO 4 3 Ca. Br 2 + 1 Co 2(SO 4)3 3 moles of Ca. SO 4 used to 1 mole of Co 2(SO 4)3 produced How many moles Co 2(SO 4)3 of can be produced if we start with 12 moles of Co. Br 3 and an excess of Ca. SO 4?

Stoichiometry – The Box Method Use the box with grams! ● ● Moles is on the bottom in this box Balance the equation first if needed! Molar mass: add masses from the periodic table Down = divide; up = multiply The ONLY way to move between compounds is from moles to moles

Acids and Bases – The Box Method Use the box with liters! ● ● Moles is on the TOP in this box Down = divide; up = multiply The ONLY way to move between compounds is from moles to moles

Stoichiometry – The Box Method ● Write chemical formulas: + and - charges MUST CANCEL ● ● ● Oxide and hydroxide are NOT the same thing Balance the equation Use the box
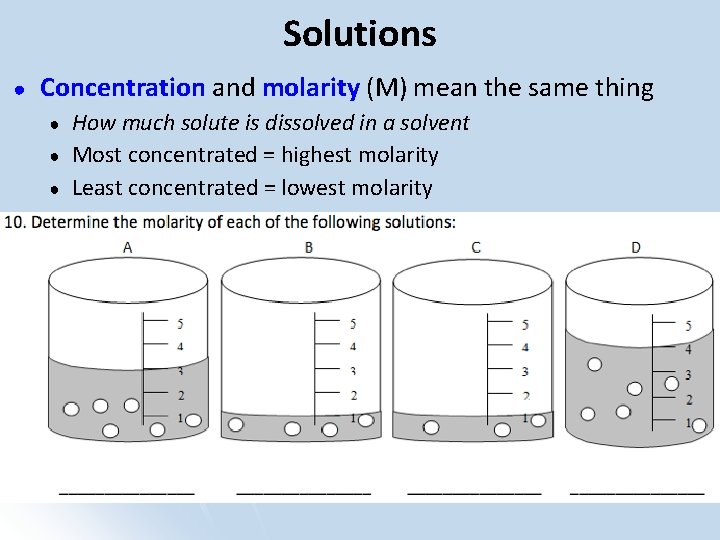
Solutions ● Concentration and molarity (M) mean the same thing ● ● ● How much solute is dissolved in a solvent Most concentrated = highest molarity Least concentrated = lowest molarity

Solutions – Freezing Point ● Dissociation factor: how many particles a solute will break into in water ● ● ● Ionic (metal + nonmetal) = breaks apart ● Circle any polyatomic ions – the group stays together! Covalent (nonmetals) = stays together = df of 1 Freezing point calculations use molality (mol divided by kg)

Solutions – Solubility Graph ● ● Solubility is temperature dependent Saturated = contains the maximum amount of solute at that temperature ● ● Any point on the curve Precipitate = solid that forms

Acids and Bases – p. H Calculations ● ● Use the flow chart! Acidic, basic, or neutral? ● Always find the p. H to tell ● p. H less than 7 = acidic ● p. H greater than 7 = basic

Acids and Bases – Neutralization Reactions ● Double replacement reactions ● ● ● One product is ALWAYS water (H 2 O) CIRCLE polyatomic ions – they stay together Formulas: + and – charges must cancel


Solutions Example #1 What is the molarity of a solution in which 10. 0 g of silver nitrate is dissolved in 500. 0 m. L of solution? Now try problem #12 in your pink review packet!

Solutions Example #1 What is the molarity of a solution in which 10. 0 g of silver nitrate is dissolved in 500. 0 m. L of solution? Now try problem #12 in your pink review packet!

Solutions Example #2 What is the boiling point of a solution of 48. 3 g of Li. Cl in 450 g of water? Now try problem #24 in your pink review packet!

Solutions Example #3 A saturated solution of sodium nitrate is cooled from 60 o. C to 20 o. C. How many grams of precipitate form? Now try problem #28 in your pink review packet!

Acids and Bases Example #1 Name the following and determine whether the compound is an acid, a base, or neither one. Anion Acid Name Ending -ide hydro-(stem)-ic acid -ite (stem)-ous acid -ate (stem)-ic acid a. HCl b. Be(OH)2 Now try problem #33 in your pink review packet!

Acids and Bases Example #2 Write the formulas of the following compounds. a. Nitrous acid Anion Acid Name Ending -ide hydro-(stem)-ic acid -ite (stem)-ous acid -ate (stem)-ic acid b. Magnesium hydroxide c. Chloric acid Now try problem #34 in your pink review packet!

Acids and Bases Example #3 Calculate the p. H of a solution in which the [OH-] = 3. 6 x 10 -6. Is the solution acidic, basic, or neutral? Now try problem #39 a in your pink review packet!
![Acids and Bases Example #4 Calculate the [H+] of a solution in which the Acids and Bases Example #4 Calculate the [H+] of a solution in which the](http://slidetodoc.com/presentation_image_h2/9fa8ef6cc232fc7bbe9d31a43de854d6/image-19.jpg)
Acids and Bases Example #4 Calculate the [H+] of a solution in which the p. OH = 7. 2. Now try problem #40 a in your pink review packet!

Acids and Bases Example #5 Write the complete balanced neutralization reaction for the reaction of phosphoric acid and barium hydroxide. Now try problems #43 -44 in your pink review packet!

Acids and Bases Example #6 A 45 m. L solution of H 2 SO 4 is completely neutralized by 22 m. L of 1. 25 M Na. OH. What is the molarity of the H 2 SO 4 solution? Moles (mol) Molarity (M) Liters (L) Now try problem #45 in your pink review packet!

Acids and Bases Example #7 What is the molarity of H 2 SO 4 if 55 m. L is neutralized by 1. 5 M Na. OH according to the curve below? Moles (mol) Molarity (M) Liters (L) Now try problem #47 in your pink review packet!

Acids and Bases Practice A 60 m. L solution of HCl is completely neutralized by 22 m. L of 0. 50 M Ba(OH)2. What is the molarity of the HCl solution? Moles (mol) Molarity (M) Liters (L) Now try problem #45 in your pink review packet!
- Slides: 23