Semantic Data Capture Initiative Hemant Shah M D












- Slides: 34

Semantic Data Capture Initiative Hemant Shah M. D. , M. Surg. Sr. Research Informatician Henry Ford Health System hshah 2@hfhs. org

What is Semantic Data Capture Initiative (SDCI)? n A project to develop decision support capabilities combined with structured data capture for Care. Plus NG, and its evaluation n Under a TATRC (Do. D) supervised grant n All code is available as Open Source (EPL) n Period of Performance q From April 2008 to October 2010

The Team n Investigators: q R. David Allard, MD (PI) q Hemant Shah, MD (Co-PI) q Patricia Williams q Ganesh Krishnan (Lead Developer) n Support from: q CPNG development team q Rich Vollmerhausen q CSRI Research Staff q CSRI Training Staff q Gloria Whitten q Marie Mc. Lenaghan n External Adviser: q Prakash Nadkarni MD

Goal n Provide Data Capture in Care. Plus EMR, which is: q Structured q Standard Based q Semantic n Encourage point-of-care data entry with Decision Support as an incentive for the clinician

Hypothesis Clinical documentation templates that leverage metadata with controlled medical vocabularies and interoperate with clinical decision support can be integrated into key ambulatory care processes in a manner acceptable to clinicians and support staff

Components of SDCI n Research Component n Software Development Component n Knowledge Development Component n Evaluation Component

Research Component n Structured Data Entry Literature Review n Guideline Systems Comparative Analysis n Controlled Medical Vocabulary Analysis q Criteria specification for Vocabularies for CPNG and Decision Support q Examine Vocabularies n n n n SNOMED-CT LOINC ICD-9 CPT NCI Thesaurus UMLS Metathesaurus MEDCIN 3. 0 q Application of Criteria n Industry Scan of existing systems

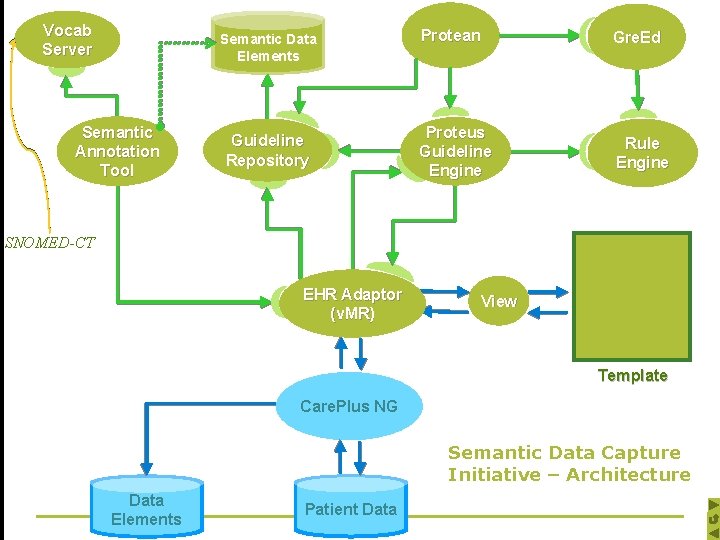
Development Component n Protean – Guideline Authoring Tool n Proteus Guideline Engine n Greed – Rule Authoring tool n Semantic Annotation Tool n Interactions with CPNG q Metadata Repository q Patient Record

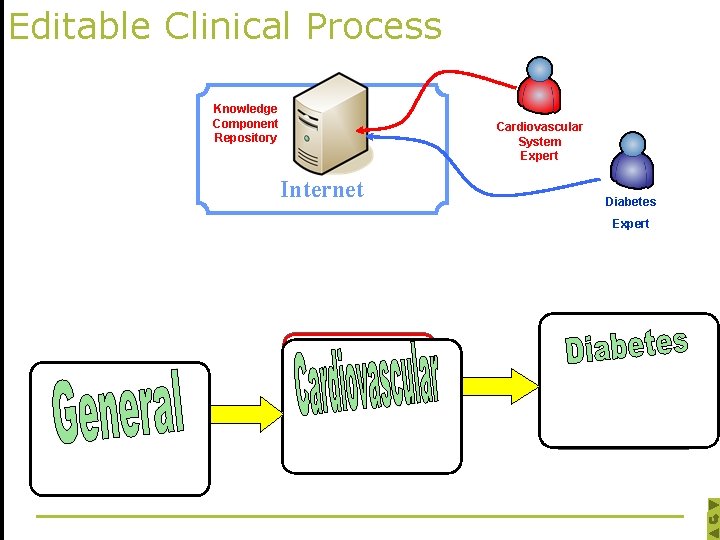
Editable Clinical Process

Editable Clinical Process

Editable Clinical Process Knowledge Component Repository Cardiovascular System Expert Internet Diabetes Expert

Proteus may be used for… n Creating executable clinical guidelines to provide decision-support to clinicians about individual patients n Creating process-oriented EMRs with integrated clinical decision-support

Proteus Model Contains… n A specification of an architecture for: q KCs q Executable Workflows (Guidelines) built with KCs q Tools and Systems to handle them n A graphical language for Workflows q Human & machine readable q Proteus Graphical Language - PGL

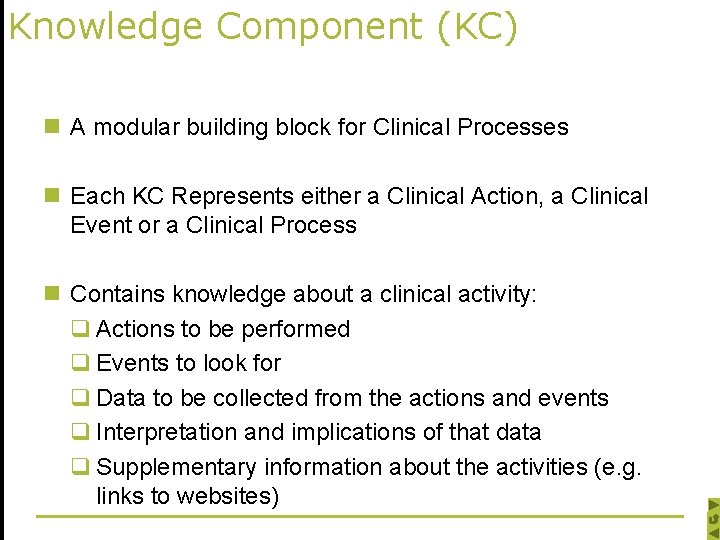
Knowledge Component (KC) n A modular building block for Clinical Processes n Each KC Represents either a Clinical Action, a Clinical Event or a Clinical Process n Contains knowledge about a clinical activity: q Actions to be performed q Events to look for q Data to be collected from the actions and events q Interpretation and implications of that data q Supplementary information about the activities (e. g. links to websites)

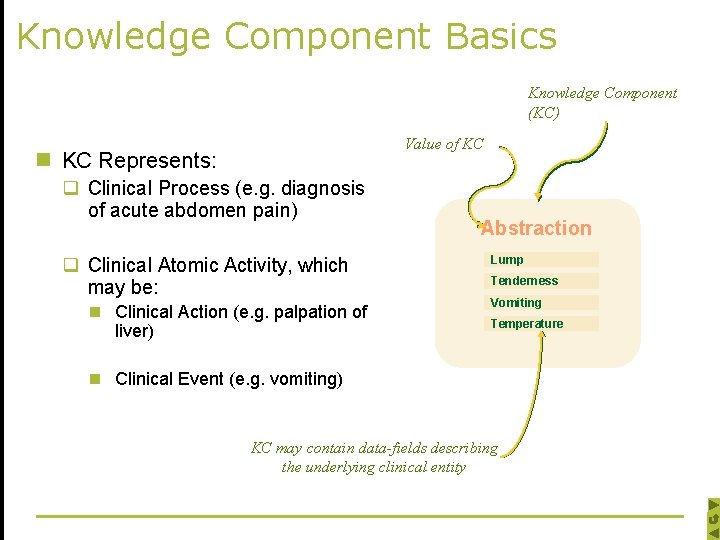
Knowledge Component Basics Knowledge Component (KC) Value of KC n KC Represents: q Clinical Process (e. g. diagnosis of acute abdomen pain) q Clinical Atomic Activity, which may be: n Clinical Action (e. g. palpation of liver) Abstraction Lump Tenderness Vomiting Temperature n Clinical Event (e. g. vomiting) KC may contain data-fields describing the underlying clinical entity

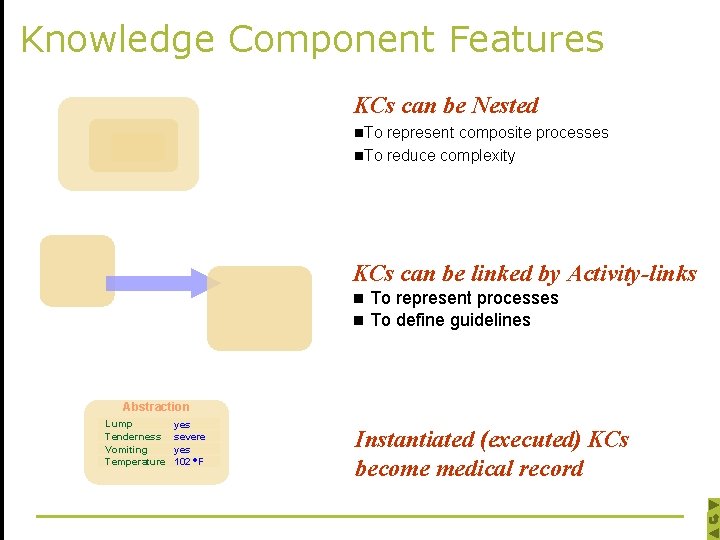
Knowledge Component Features KCs can be Nested n. To represent composite processes n. To reduce complexity KCs can be linked by Activity-links n To represent processes n To define guidelines Abstraction Lump Tenderness Vomiting Temperature yes severe yes 102 F Instantiated (executed) KCs become medical record

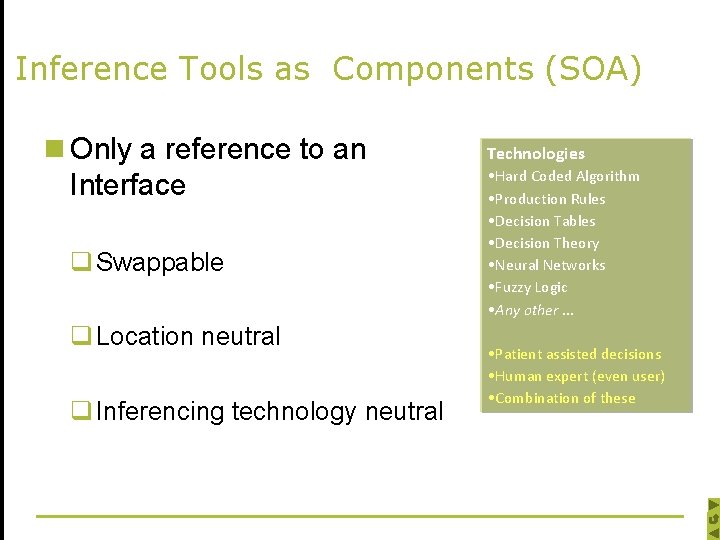
Inference Tools as Components (SOA) n Only a reference to an Interface q Swappable q Location neutral q Inferencing technology neutral Technologies • Hard Coded Algorithm • Production Rules • Decision Tables • Decision Theory • Neural Networks • Fuzzy Logic • Any other … • Patient assisted decisions • Human expert (even user) • Combination of these

Inference Tools as Components n Two Types of Inference Tools q Inference tool for Abstraction q Inference tool for Action

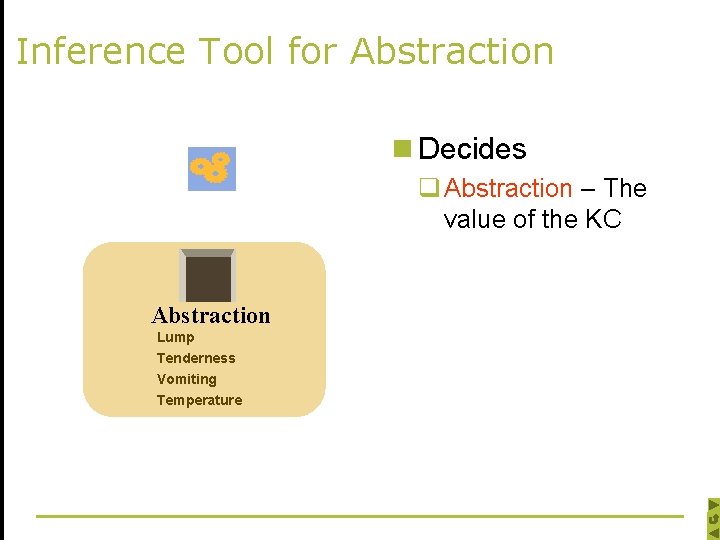
Inference Tool for Abstraction n Decides q Abstraction – The value of the KC Abstraction Lump Tenderness Vomiting Temperature

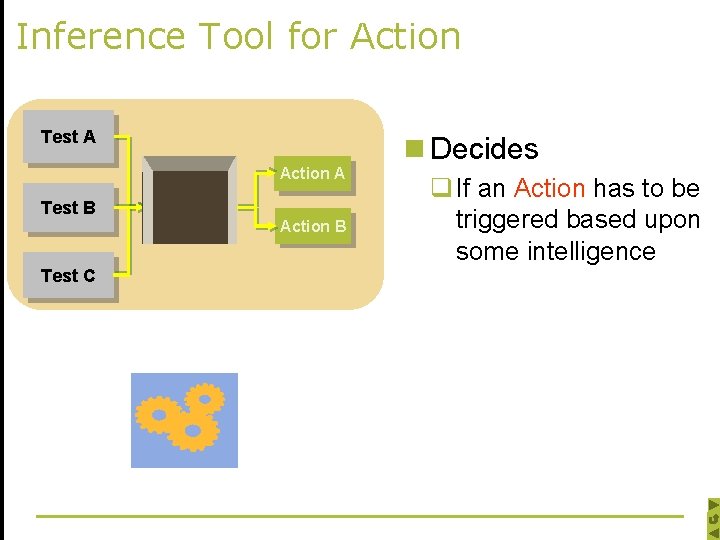
Inference Tool for Action Test A Action A Test B Test C Action B n Decides q If an Action has to be triggered based upon some intelligence

Protean – An Integrated Process Authoring Tool A Demo

Vocab Server Semantic Data Patient Data Elements Semantic Annotation Tool Guideline Repository Protean Gre. Ed Proteus Guideline Engine Rule Engine SNOMED-CT EHR Adaptor (v. MR) View Template Care. Plus NG Semantic Data Capture Initiative – Architecture Data Elements Patient Data

What is Greed? n A tool to author and edit rules q Easy to use graphical representation of rules q Drag and drop is all you need q Internal rule syntax inspired by LISP q Ability to create rules in multiple languages, e. g. , Arden Syntax, Java, Rule. ML, Jess, JBoss Rules etc. q Semantic and Completeness checks on rules q Allows testing of rules from within the environment q Currently in use by Protean q Future Plans: n Use ISO/IEC 11179 data elements for conditions and inferences n Extensibility – New logical or math operations can be added n Rule repository related features n To be made available as an independent Rules system

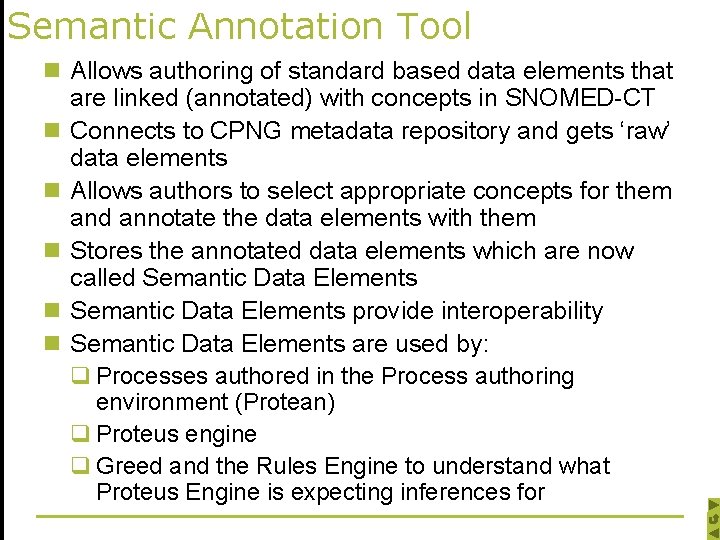
Semantic Annotation Tool n Allows authoring of standard based data elements that are linked (annotated) with concepts in SNOMED-CT n Connects to CPNG metadata repository and gets ‘raw’ data elements n Allows authors to select appropriate concepts for them and annotate the data elements with them n Stores the annotated data elements which are now called Semantic Data Elements n Semantic Data Elements provide interoperability n Semantic Data Elements are used by: q Processes authored in the Process authoring environment (Protean) q Proteus engine q Greed and the Rules Engine to understand what Proteus Engine is expecting inferences for

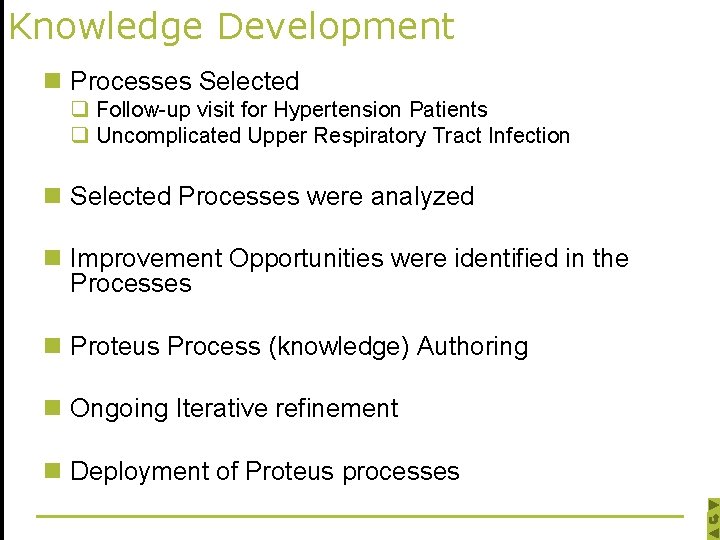
Knowledge Development n Processes Selected q Follow-up visit for Hypertension Patients q Uncomplicated Upper Respiratory Tract Infection n Selected Processes were analyzed n Improvement Opportunities were identified in the Processes n Proteus Process (knowledge) Authoring n Ongoing Iterative refinement n Deployment of Proteus processes

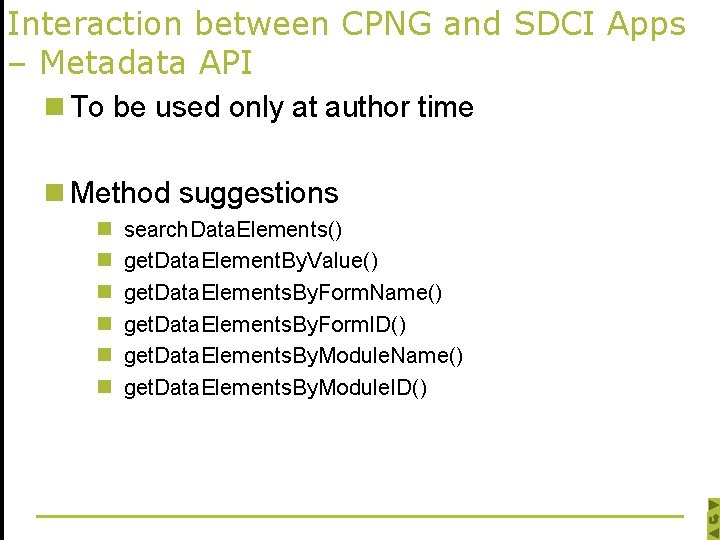
Interaction between CPNG and SDCI Apps – Metadata API n To be used only at author time n Method suggestions n n n search. Data. Elements() get. Data. Element. By. Value() get. Data. Elements. By. Form. Name() get. Data. Elements. By. Form. ID() get. Data. Elements. By. Module. Name() get. Data. Elements. By. Module. ID()

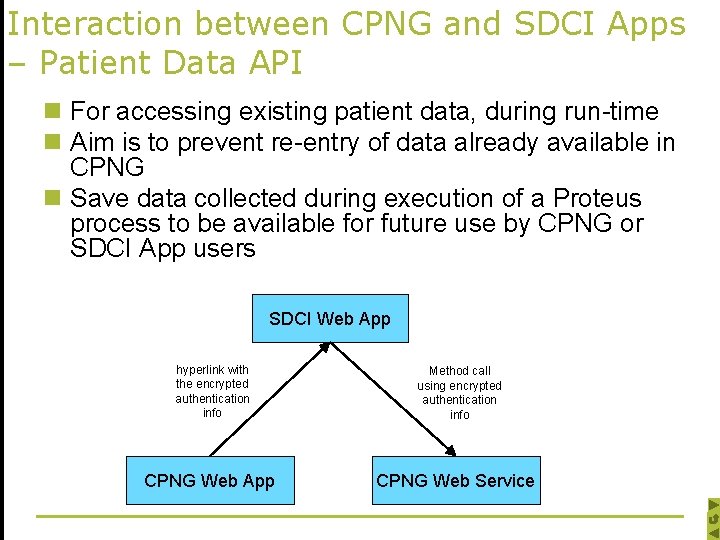
Interaction between CPNG and SDCI Apps – Patient Data API n For accessing existing patient data, during run-time n Aim is to prevent re-entry of data already available in CPNG n Save data collected during execution of a Proteus process to be available for future use by CPNG or SDCI App users SDCI Web App hyperlink with the encrypted authentication info CPNG Web App Method call using encrypted authentication info CPNG Web Service

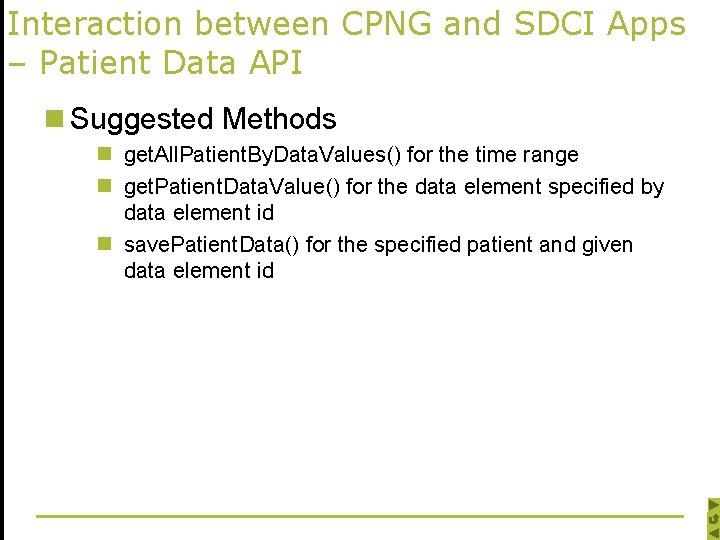
Interaction between CPNG and SDCI Apps – Patient Data API n Suggested Methods n get. All. Patient. By. Data. Values() for the time range n get. Patient. Data. Value() for the data element specified by data element id n save. Patient. Data() for the specified patient and given data element id

thank you

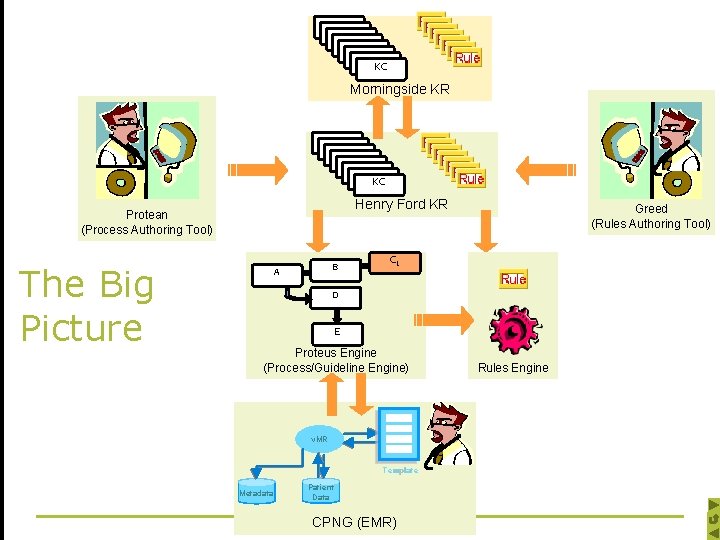
KC Morningside KR KC Henry Ford KR Protean (Process Authoring Tool) The Big Picture B A Greed (Rules Authoring Tool) CC 1 D E Proteus Engine (Process/Guideline Engine) v. MR Template Metadata Patient Data CPNG (EMR) Rules Engine

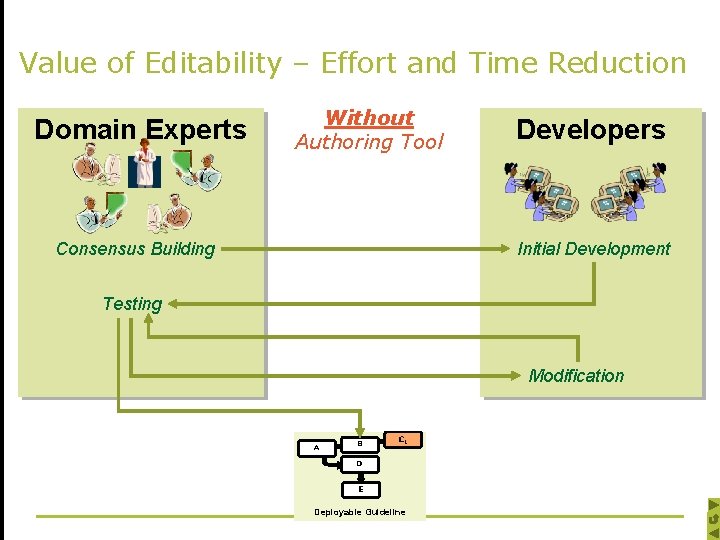
Value of Editability – Effort and Time Reduction Domain Experts Without Authoring Tool Consensus Building Developers Initial Development Testing Modification A B CC 1 D E Deployable Guideline

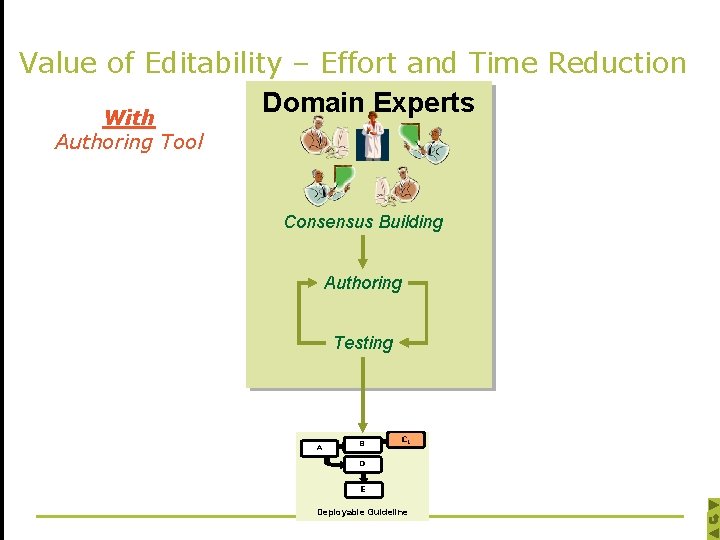
Value of Editability – Effort and Time Reduction Domain Experts With Authoring Tool Consensus Building Authoring Testing A B CC 1 D E Deployable Guideline

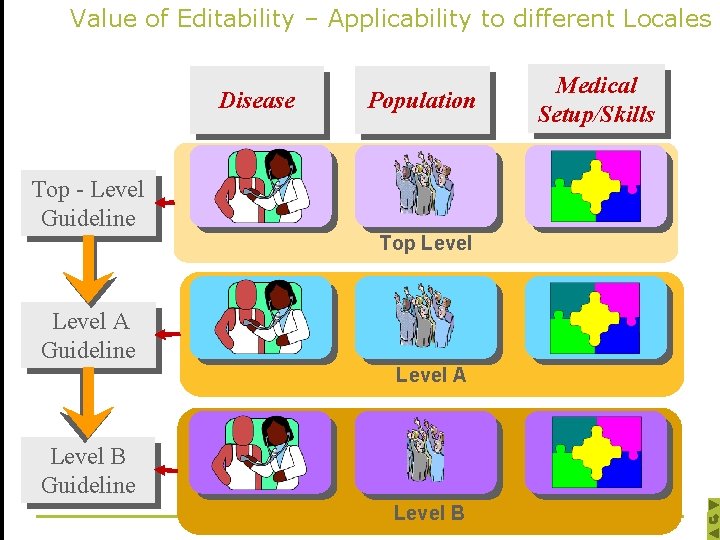
Value of Editability – Applicability to different Locales Disease Population Top - Level Guideline Top Level A Guideline Level A Level B Guideline Level B Medical Setup/Skills

Evaluation