Self Ionization of Water and the p H





![Estimating p. H from [H 3 O+] The p. H of a solution can Estimating p. H from [H 3 O+] The p. H of a solution can](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-8.jpg)
![Estimating p. H from + [H 3 O ] The p. H of a Estimating p. H from + [H 3 O ] The p. H of a](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-9.jpg)
![Estimating p. H from + [H 3 O ] The p. H of a Estimating p. H from + [H 3 O ] The p. H of a](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-10.jpg)
![Estimating p. H from + [H 3 O ] The p. H of a Estimating p. H from + [H 3 O ] The p. H of a](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-11.jpg)
![Estimating [H 3 O+] from p. H Example: p. H = 9. 0 So, Estimating [H 3 O+] from p. H Example: p. H = 9. 0 So,](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-12.jpg)
![Estimating [H 3 O+] from p. H You try: p. H = 7. 0 Estimating [H 3 O+] from p. H You try: p. H = 7. 0](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-13.jpg)
- Slides: 13

Self Ionization of Water and the p. H Scale

Ionization of Water will naturally break down into equal ions in water: ◦ 2 H 2 O H 3 O+ (aq) + OH- (aq) ◦ This is called the ionization of water.

p. H Scale • scale used by chemists to measure the acidity of a substance; a measure of the amount of H+ in the solution. • scale goes from 0 – 14 NEUTRAL 7 0 ACIDIC OH- < H 3 O+ (We are counting how many hydronium ions are in a solution!) OH- = H 3 O+ 14 BASIC OH- > H 3 O+ (We are counting how many hydroxide ions are in a solution!)

p. H Scale p. H describes a balance between hydroxide ions (OH-) and hydronium ions (H 3 O+) ◦ Note: [H+] and [H 3 O+] are equivalent! We will use them interchangeably. ◦ p. H stands for “parts hydrogen ion” ◦ p. OH stands for “parts hydroxide ion”

Is It An Acid or a Base? 1. p. H = 4 2. p. H = 10 3. p. H = 7

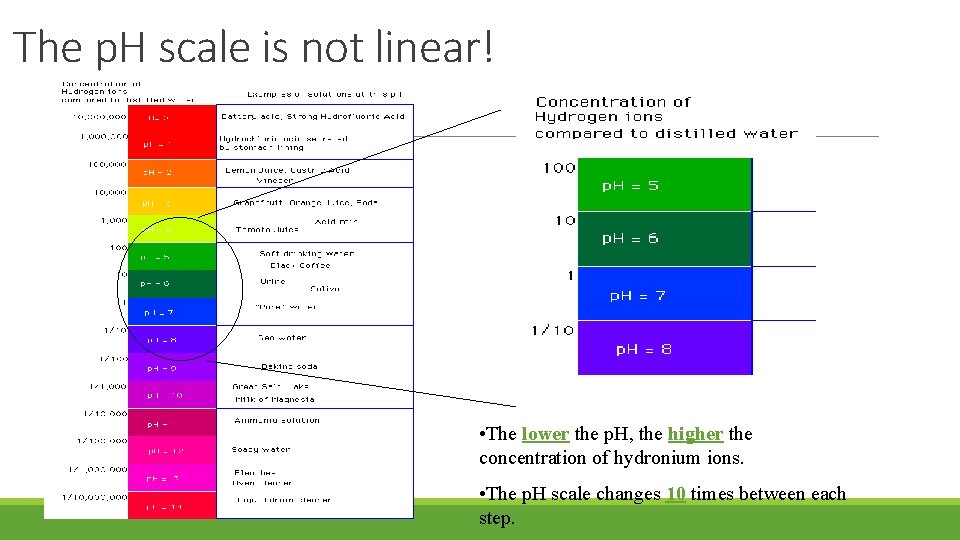
The p. H scale is not linear! • The lower the p. H, the higher the concentration of hydronium ions. • The p. H scale changes 10 times between each step.

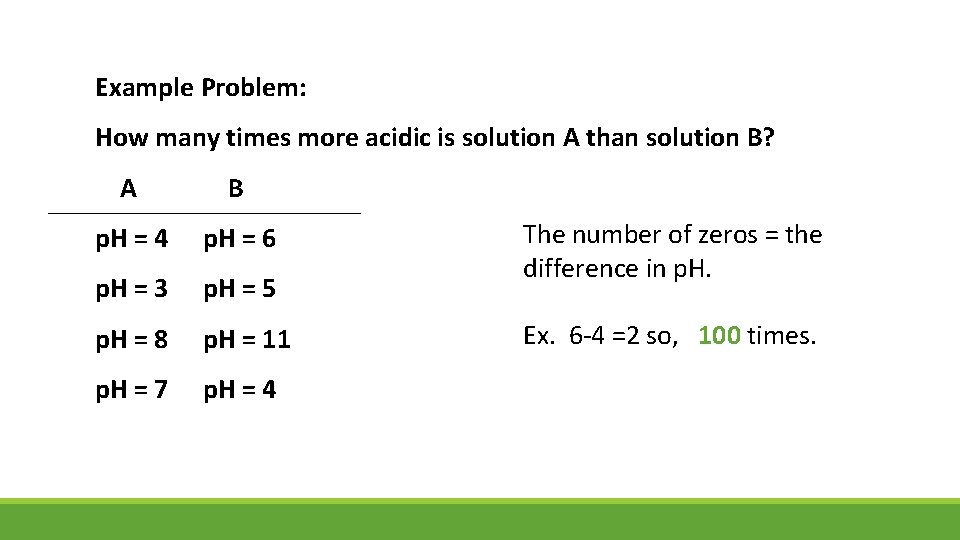
Example Problem: How many times more acidic is solution A than solution B? A B p. H = 4 p. H = 6 p. H = 3 p. H = 5 p. H = 8 p. H = 11 p. H = 7 p. H = 4 The number of zeros = the difference in p. H. Ex. 6 -4 =2 so, 100 times.
![Estimating p H from H 3 O The p H of a solution can Estimating p. H from [H 3 O+] The p. H of a solution can](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-8.jpg)
Estimating p. H from [H 3 O+] The p. H of a solution can be estimated by looking at the concentration of hydronium (H 3 O+). The exponent is the estimated p. H of the substance! Example: [H 3 O+] = 1. 0 x 10 -3 M p. H = 3
![Estimating p H from H 3 O The p H of a Estimating p. H from + [H 3 O ] The p. H of a](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-9.jpg)
Estimating p. H from + [H 3 O ] The p. H of a solution can be estimated by looking at the concentration of hydronium (H 3 O+). Example: [H 3 O+] = 1. 0 x 10 -12 M p. H = 12
![Estimating p H from H 3 O The p H of a Estimating p. H from + [H 3 O ] The p. H of a](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-10.jpg)
Estimating p. H from + [H 3 O ] The p. H of a solution can be estimated by looking at the concentration of hydronium (H 3 O+). Example: [H 3 O+] = 3. 1 x 10 -12 M
![Estimating p H from H 3 O The p H of a Estimating p. H from + [H 3 O ] The p. H of a](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-11.jpg)
Estimating p. H from + [H 3 O ] The p. H of a solution can be estimated by looking at the concentration of hydronium (H 3 O+). Example: [H 3 O+] = 3. 1 x 10 -12 M p. H = a little less than 12
![Estimating H 3 O from p H Example p H 9 0 So Estimating [H 3 O+] from p. H Example: p. H = 9. 0 So,](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-12.jpg)
Estimating [H 3 O+] from p. H Example: p. H = 9. 0 So, [H 3 O+] = 1. 0 x 10 -9 M
![Estimating H 3 O from p H You try p H 7 0 Estimating [H 3 O+] from p. H You try: p. H = 7. 0](https://slidetodoc.com/presentation_image_h/08ccb9275ae9d7738648243f215f6483/image-13.jpg)
Estimating [H 3 O+] from p. H You try: p. H = 7. 0 p. H = 2. 0 p. H = 8. 0