Selective production of acetone during continuous synthesis gas


- Slides: 21

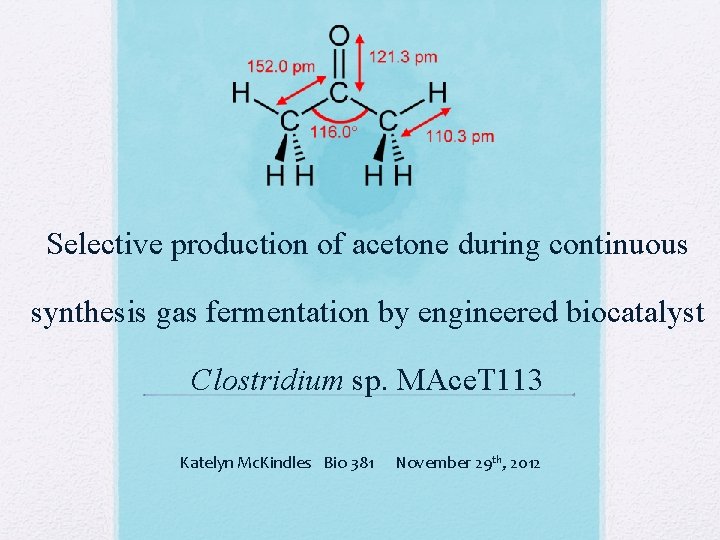
Selective production of acetone during continuous synthesis gas fermentation by engineered biocatalyst Clostridium sp. MAce. T 113 Katelyn Mc. Kindles Bio 381 November 29 th, 2012

Experimental Aims • To engineer an acetogen biocatalyst capable of fermenting synthesis gas blend to acetone as the only liquid carbonaceous product

Acetone • Acetone is used in the industry as an organic solvent • • Currently produced from propylene and benzene mixture resulting from petroleum cracking and refining processes • • • Thinning fiberglass resin Metal degreaser Paint component Organic reaction solvent (Jones Oxidation) Acne treatments Nail polish remover cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. Expensive process- ~$1000/ton

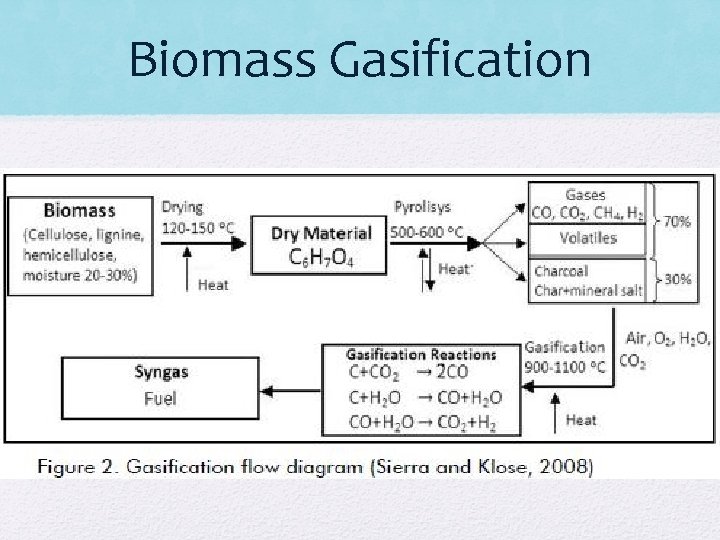
Synthesis Gas Fermentation The structural materials that plants produce to form the cell walls, leaves, stems, stalks, and woody portions of biomass are composed mainly of three biobased chemicals called cellulose, hemicellulose, and lignin. Together, they are called lignocellulose, a composite material of rigid cellulose fibers embedded in a cross-linked matrix of lignin and hemicellulose that bind the fibers. Lignocellulose material is by necessity resistant to physical, chemical, and biological attack, but it is of interest to biorefining because the cellulose and hemicellulose can be broken down through a process called hydrolysis to produce fermentable, simple sugars. Lignocellulosic biomass is often a waste material of the food processing and forest products industries that may be locally, readily available at low cost. Biomass gasification is a high-temperature process (600 to 1000 o. C) to decompose the complex hydrocarbons of biomass into simpler gaseous molecules, primarily hydrogen, carbon monoxide, and carbon dioxide.

Biomass Gasification

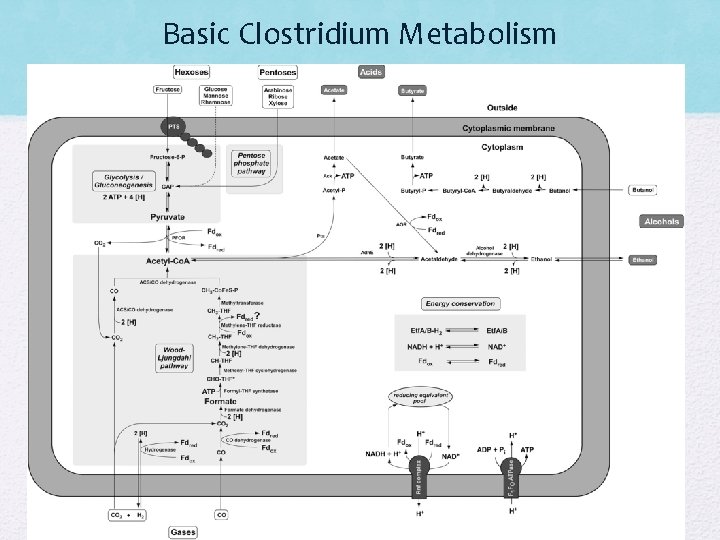
Clostridium • Clostridium species are Grampositive, rod-shaped, sporeformers. These generally obligate anaerobes are ubiquitous saprophytes or part of our normal flora. • Clostridia employ butyric fermentation pathways to generate energy and, as a result, often produce a foul odor. • Novel metabolic pathways: Wood-Ljungdahl pathway, Solventgenesis, ABE pathway • Experiment utilized Clostridium sp. MT 896 as it had a tolerence of acetone at 2. 5 mol l-1 • Mutant can only produce Ethanol and acetate (no butanol pathway)

Basic Clostridium Metabolism

Acetone-Butanol-Ethanol Process • Among the saccharolytic butyric acid-producing clostridia, there a number of species capable of producing significant amounts of neutral solvents during the later stages of batch fermentation. • As the culture enters the stationary growth phase, the metabolism of the cells undergo a shift to solvent production • Research including this pathway fell off after World War II, when feed stocks such as maize and molasses were in high demand • Byproducts tend to be highly toxic, produced in low concentrations • Butanol is toxic at low concentrations, limiting the level of solvent in the final fermentation broth around 2% • Acetone sensitivity is about 86 mmol l-1


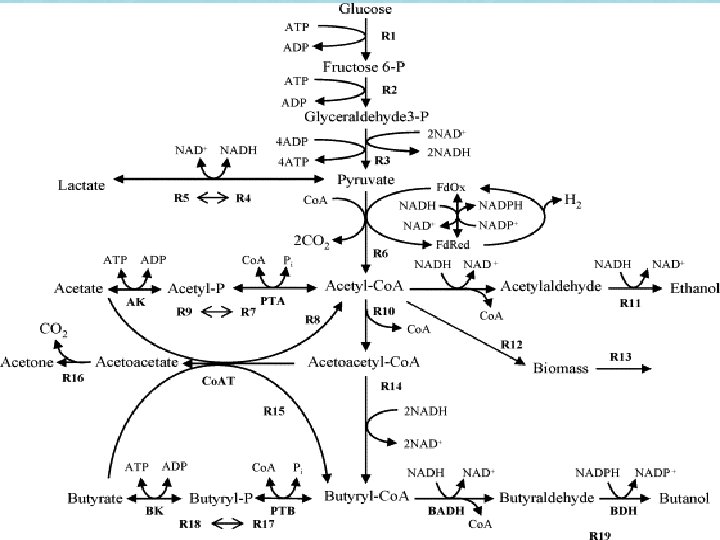
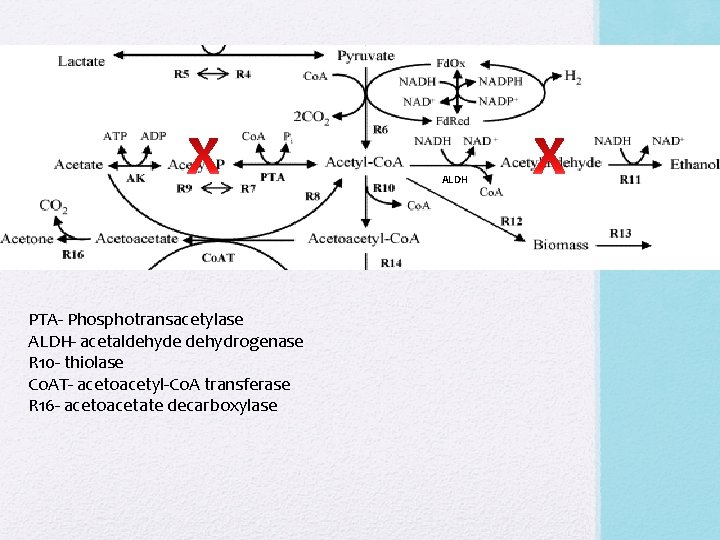
Gene Inactivation • Inactivation of phosphotransacetylase which prevents production and accumulation of acetic acid (leads to growth inhibition) and inactivation of acetaldehyde dehydrogenase to prevent acetaldehyde production • Genes inactivated through the introduction of synthesized suicidal vectors (1) p. MTerm(B)pta 23 and (2) p. MTcat_aldh 13 • (1) Uses Erythromycin resistance gene erm(B) from Moorella thermoacetica for screening. Contains genes for thiolase (thio) and hydroxymethyglutaryl-Co. A synthase (hmg. Co. As), as well as a fragment of pta from Clostridium ljungdahlii • (2) Uses Chloramphenicol acetyltransferase (resistance) gene cat from M. thermoacetica for screening. Contains genes for hydroxymethylglutaryl. Co. A lyase (hmg. Co. Al) and acetoacetate decarboxylase (adc), as well as a fragment of aldh from Cl. Ljungdahlii • p. UC 19 used as DNA backbone. From E. coli JM 109

Experiment Growth Conditions • Anaerobic Chamber or anaerobic Vacu-Quick Jars • Syngas (60% CO, 40% H 2) • Cell exposure to nonsyngas conditions caused sporulation • Incubated at 36°C • Antibiotics used to grow recombinants: • Choloramphenicol • Erythromycin

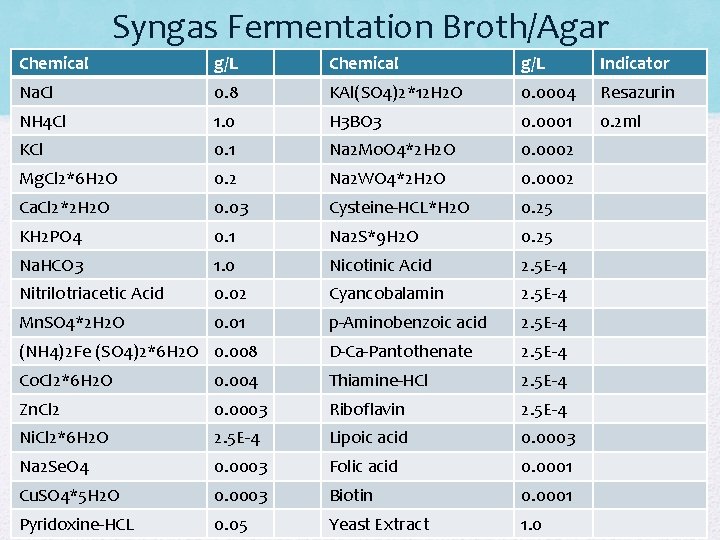
Syngas Fermentation Broth/Agar Chemical g/L Indicator Na. Cl 0. 8 KAl(SO 4)2*12 H 2 O 0. 0004 Resazurin NH 4 Cl 1. 0 H 3 BO 3 0. 0001 0. 2 ml KCl 0. 1 Na 2 Mo. O 4*2 H 2 O 0. 0002 Mg. Cl 2*6 H 2 O 0. 2 Na 2 WO 4*2 H 2 O 0. 0002 Ca. Cl 2*2 H 2 O 0. 03 Cysteine-HCL*H 2 O 0. 25 KH 2 PO 4 0. 1 Na 2 S*9 H 2 O 0. 25 Na. HCO 3 1. 0 Nicotinic Acid 2. 5 E-4 Nitrilotriacetic Acid 0. 02 Cyancobalamin 2. 5 E-4 Mn. SO 4*2 H 2 O 0. 01 p-Aminobenzoic acid 2. 5 E-4 (NH 4)2 Fe (SO 4)2*6 H 2 O 0. 008 D-Ca-Pantothenate 2. 5 E-4 Co. Cl 2*6 H 2 O 0. 004 Thiamine-HCl 2. 5 E-4 Zn. Cl 2 0. 0003 Riboflavin 2. 5 E-4 Ni. Cl 2*6 H 2 O 2. 5 E-4 Lipoic acid 0. 0003 Na 2 Se. O 4 0. 0003 Folic acid 0. 0001 Cu. SO 4*5 H 2 O 0. 0003 Biotin 0. 0001 Pyridoxine-HCL 0. 05 Yeast Extract 1. 0

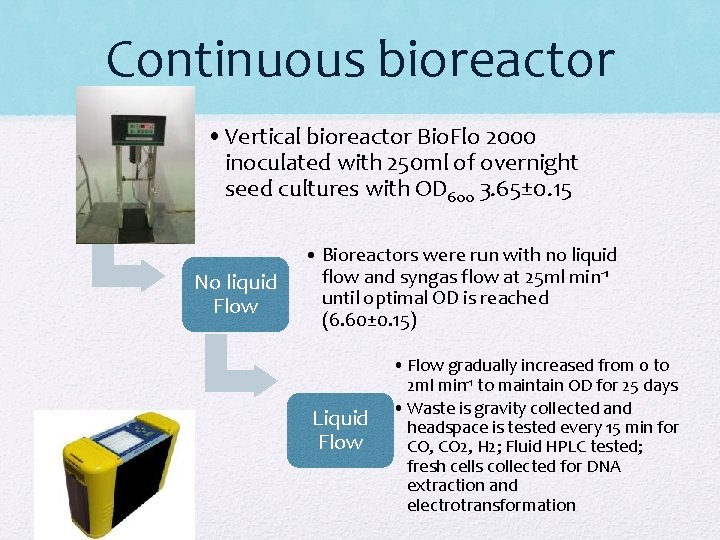
Continuous bioreactor • Vertical bioreactor Bio. Flo 2000 inoculated with 250 ml of overnight seed cultures with OD 600 3. 65± 0. 15 No liquid Flow • Bioreactors were run with no liquid flow and syngas flow at 25 ml min-1 until optimal OD is reached (6. 60± 0. 15) Liquid Flow • Flow gradually increased from 0 to 2 ml min-1 to maintain OD for 25 days • Waste is gravity collected and headspace is tested every 15 min for CO, CO 2, H 2; Fluid HPLC tested; fresh cells collected for DNA extraction and electrotransformation

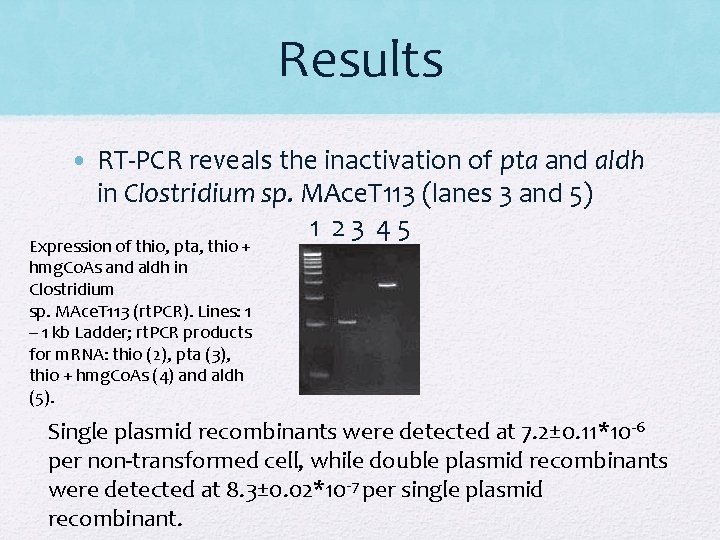
Results • RT-PCR reveals the inactivation of pta and aldh in Clostridium sp. MAce. T 113 (lanes 3 and 5) 1 23 45 Expression of thio, pta, thio + hmg. Co. As and aldh in Clostridium sp. MAce. T 113 (rt. PCR). Lines: 1 – 1 kb Ladder; rt. PCR products for m. RNA: thio (2), pta (3), thio + hmg. Co. As (4) and aldh (5). Single plasmid recombinants were detected at 7. 2± 0. 11*10 -6 per non-transformed cell, while double plasmid recombinants were detected at 8. 3± 0. 02*10 -7 per single plasmid recombinant.

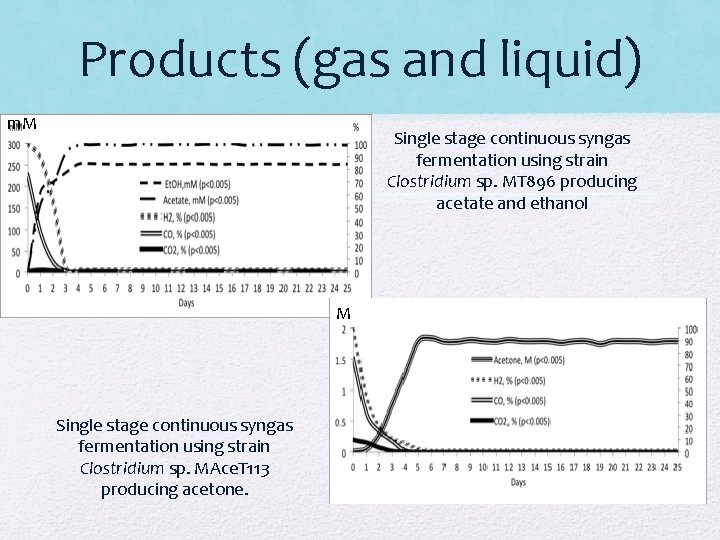
Products (gas and liquid) m. M Single stage continuous syngas fermentation using strain Clostridium sp. MT 896 producing acetate and ethanol M Single stage continuous syngas fermentation using strain Clostridium sp. MAce. T 113 producing acetone.

Discussion • The use of syngas to produce acetone does not depend on food or petroleum market • Acetone yield 20 x of that achieved during conventional ABE fermentation • Continuous bioreactor reduces cost due to intercyclic maintenance • The addition of a solar panel to the system would enhance hydrogen production for the process needs that would enhance energy recovery when coupled with water electrolysis

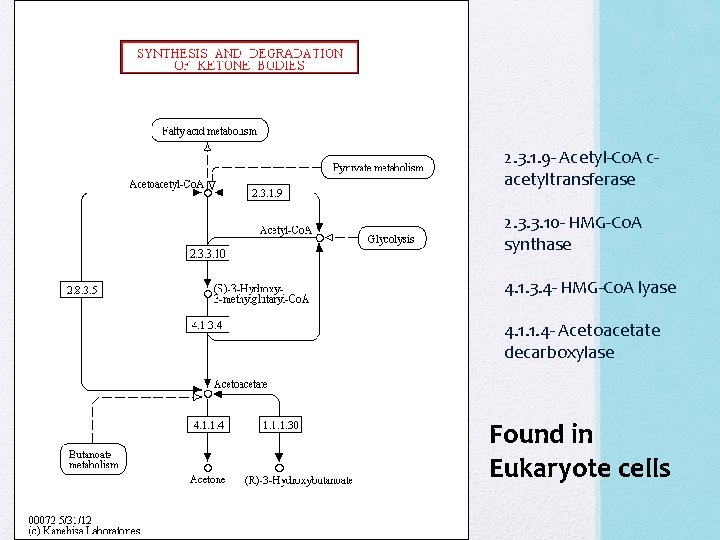
ALDH PTA- Phosphotransacetylase ALDH- acetaldehyde dehydrogenase R 10 - thiolase Co. AT- acetoacetyl-Co. A transferase R 16 - acetoacetate decarboxylase

2. 3. 1. 9 - Acetyl-Co. A cacetyltransferase 2. 3. 3. 10 - HMG-Co. A synthase 4. 1. 3. 4 - HMG-Co. A lyase 4. 1. 1. 4 - Acetoacetate decarboxylase Found in Eukaryote cells

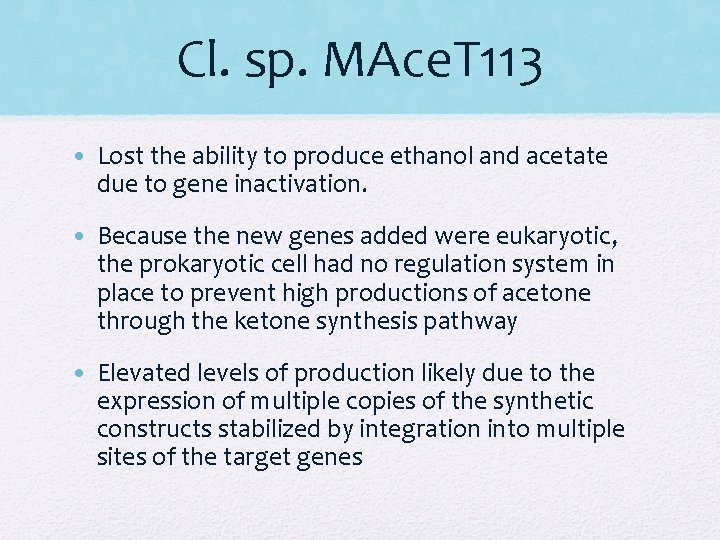
Cl. sp. MAce. T 113 • Lost the ability to produce ethanol and acetate due to gene inactivation. • Because the new genes added were eukaryotic, the prokaryotic cell had no regulation system in place to prevent high productions of acetone through the ketone synthesis pathway • Elevated levels of production likely due to the expression of multiple copies of the synthetic constructs stabilized by integration into multiple sites of the target genes

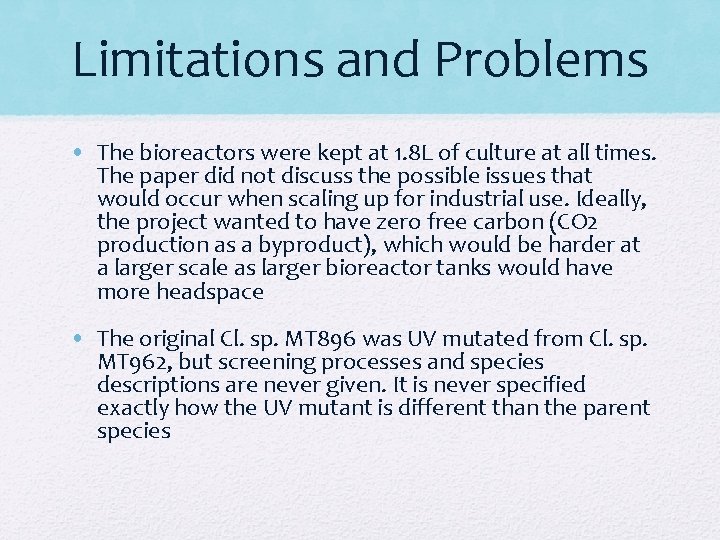
Limitations and Problems • The bioreactors were kept at 1. 8 L of culture at all times. The paper did not discuss the possible issues that would occur when scaling up for industrial use. Ideally, the project wanted to have zero free carbon (CO 2 production as a byproduct), which would be harder at a larger scale as larger bioreactor tanks would have more headspace • The original Cl. sp. MT 896 was UV mutated from Cl. sp. MT 962, but screening processes and species descriptions are never given. It is never specified exactly how the UV mutant is different than the parent species

References • “Fermentation of Lignocellulose Biomass, ” Wisconsin biorefining development initiative. www. wisbiorefine. org • Jones and Woods (1986) Acetone-Butanol Fermentation Revisited. Microbiological Reviews. 50. 4: 484 -524 • Tyurin, Kiriukhin, Berzin (2012) Electrofusion of cells of Acetogen Clostridium sp. MT 351 with erm(B) or cat in the chromosome. Journal of Biotech Research. 4: 1 -12 • “Synthesis and Degradation of Ketone Bodies, ” Kegg Pathway. http: //www. kegg. jp/keggbin/highlight_pathway? scale=1. 0&map=map 00072&keyword=ketone • “Clostridium, ” http: //www. cehs. siu. edu/fix/medmicro/clost. htm • Kopke, Held, Hujer et al. (2010) Clostridium ljungdahlii represents a microbial production platform based on syngas. PNAS 107. 29: 13087 -13092 • Berzin, Kiriukhin, Tyurin (2012) Selective production of acetone during continuous synthesis gas fermentation by engineered biocatalyst Clostridium sp. MAce. T 113. Letters in Applied Microbiology. 55: 149 -154 • Rubio, Sierra, Guerrero (2011) Gasification from waste organic material. Ing. Investig. 31: 17 -25