Sedimentation Settling efficiency in plain settling Upflow type



![Coagulation Particle sizes and treatment methods [Schematic diagram, not actual results] Chemical treatment and/or Coagulation Particle sizes and treatment methods [Schematic diagram, not actual results] Chemical treatment and/or](https://slidetodoc.com/presentation_image_h2/02c472e6f5f9f2ffe0ce4be83cec6474/image-14.jpg)













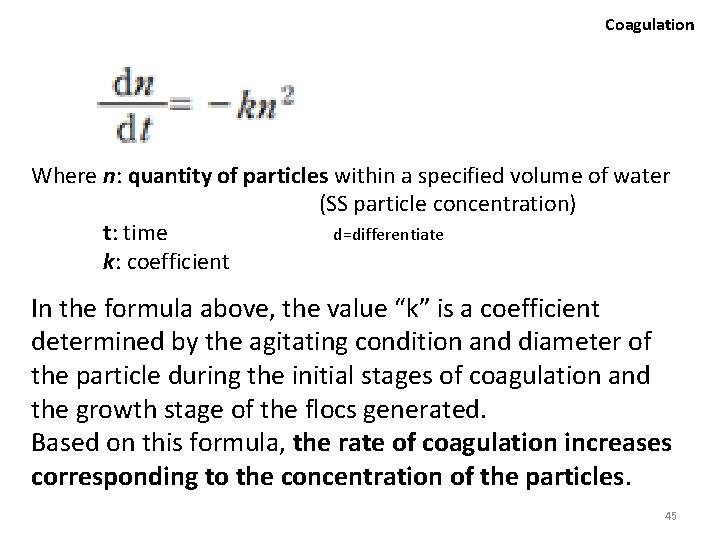





- Slides: 58

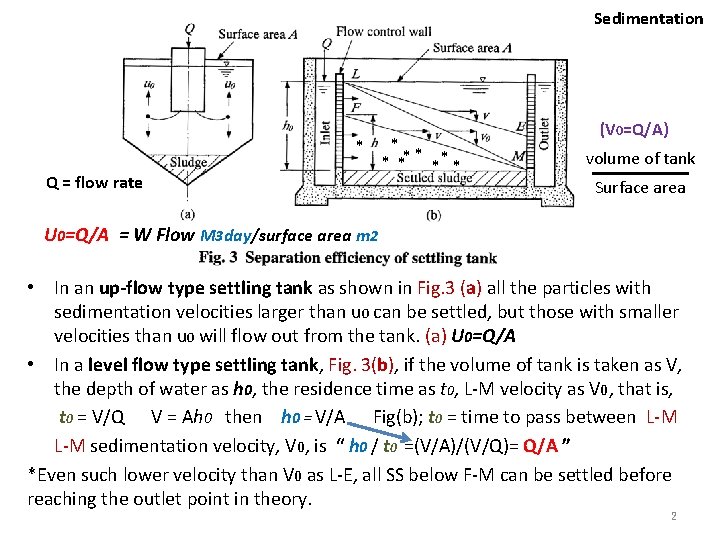
Sedimentation Settling efficiency in plain settling Up-flow type settling tank • The efficiency is determined by the settling velocity distribution of suspended solids and the up-flow rate (m 3/m 2・d) of the precipitator (clarifier) if the flow of water in the precipitator is very ideal, i. e. , • No turbulence • No short current etc. – When the separation surface area of the precipitator is taken as A and the flow rate of water as Q, then the up-flow velocity (U 0) is Q/A. U 0=Q/A • To improve the settling efficiency: – Larger surface area of A, less flow rate of Q, and increasing SS particle sedimentation rate 1

Sedimentation * Q = flow rate * * ** * (V 0=Q/A) volume of tank Surface area U 0=Q/A = W Flow M 3 day/surface area m 2 • In an up-flow type settling tank as shown in Fig. 3 (a) all the particles with sedimentation velocities larger than u 0 can be settled, but those with smaller velocities than u 0 will flow out from the tank. (a) U 0=Q/A • In a level flow type settling tank, Fig. 3(b), if the volume of tank is taken as V, the depth of water as h 0, the residence time as t 0, L-M velocity as V 0, that is, t 0 = V/Q V = Ah 0 then h 0 = V/A Fig(b); t 0 = time to pass between L-M sedimentation velocity, V 0, is “ h 0 / t 0 =(V/A)/(V/Q)= Q/A ” *Even such lower velocity than V 0 as L-E, all SS below F-M can be settled before reaching the outlet point in theory. 2

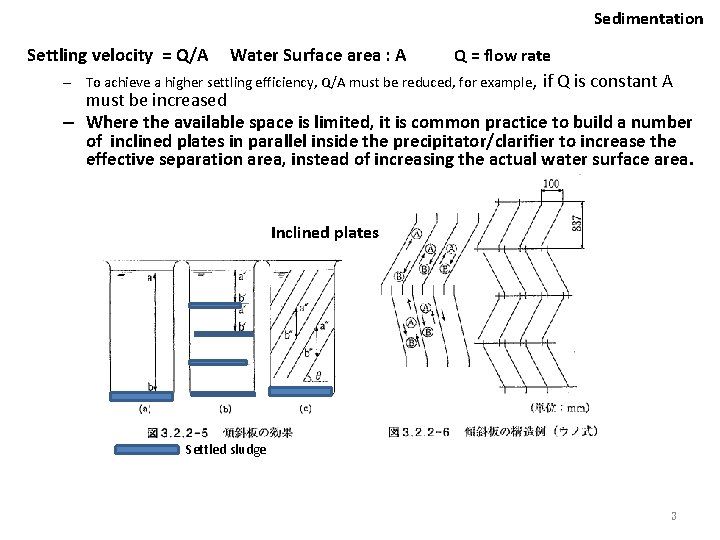
Sedimentation Settling velocity = Q/A Water Surface area : A Q = flow rate – To achieve a higher settling efficiency, Q/A must be reduced, for example, if Q is constant A must be increased – Where the available space is limited, it is common practice to build a number of inclined plates in parallel inside the precipitator/clarifier to increase the effective separation area, instead of increasing the actual water surface area. Inclined plates Settled sludge 3

Sedimentation Inclined plates (Fukuoka pref. ) Inclined pipes 4

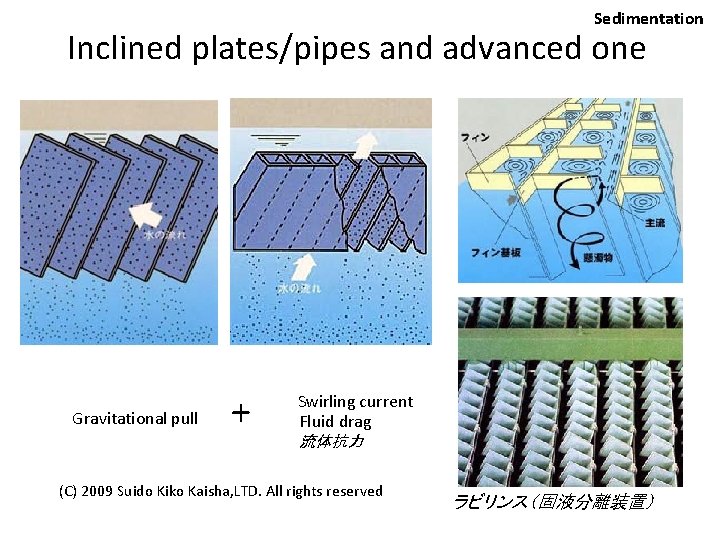
Sedimentation Inclined plates/pipes and advanced one Gravitational pull + Swirling current Fluid drag 流体抗力 (C) 2009 Suido Kiko Kaisha, LTD. All rights reserved ラビリンス(固液分離装置)

Sedimentation Q: Why they do not use Inclined-plate clarifiers for biological sludge tank? • Activated sludge treatment - bacteria – As long experience has proved bacteria and other biomass can grow on the plates, plugging or reducing the settling area. • Gradual plugging between the plates may occur in general, so clean-up is recommended. – Oily or sticky SS may also creates plugging problems between the plates. – Piled sludge on the plates may break and collapse thin Inclined-plates. 6

Sedimentation Inclined-plate clarifiers in USA 1 • The inclined-plate clarifier reduces the necessary settling depth, so it reduces the necessary settling area by up to 90% and highly effective in removing suspended solids – In conventional sedimentation, the overflow rate is used to calculate the required surface area of the sedimentation basins. So, all particles with a settling rate equal to or greater than the overflow rate will be removed. The tank depth does not affect solids removal effectiveness. Water Environment Federation, USA 2008 7

Sedimentation Inclined-plate clarifiers in USA 2 • Not usually used to clarify biological sludges because bacteria (and other biomass) can grow on the plates, plugging or reducing the settling area. • Oily ‘sticky’ solids may also create plugging problems between the plates. – Conventional sedimentation or flotation should be considered in these cases. Water Environment Federation, USA 2008 8

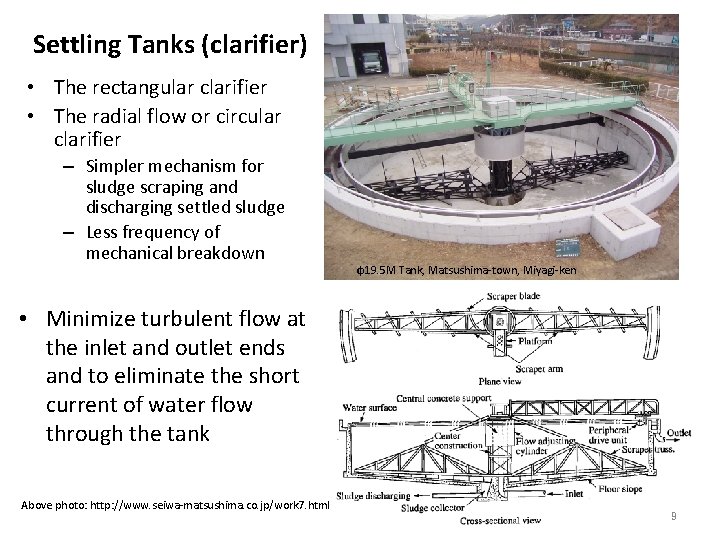
Settling Tanks (clarifier) • The rectangular clarifier • The radial flow or circular clarifier – Simpler mechanism for sludge scraping and discharging settled sludge – Less frequency of mechanical breakdown φ19. 5 M Tank, Matsushima-town, Miyagi-ken • Minimize turbulent flow at the inlet and outlet ends and to eliminate the short current of water flow through the tank Above photo: http: //www. seiwa-matsushima. co. jp/work 7. html 9

3. 2. 3 Coagulative separation 10

Coagulation sedimentation • Particles bigger than 1 - 10 μm can be separated by spontaneous settling (plain sedimentation) and/or by filtration • Particles less than 1 μm (up to 0. 001 μm) shall be applied ‘Coagulation sedimentation’ • Coagulant helps to form flocs Coagulation • Target pollution control – COD, Color, Oil etc. – Water treatment level – Floc size and settling velocity • • Cost and expenses Sludge generation Management/control Fate of the coagulant Coagulant must be cheap and must not be hazardous substances 11

Coagulation The inorganic flocculating agents positively charged, such as Fe/Al, neutralize the particle surface electric charges, however, High-polymer coagulants induce coagulation by bridging particles in addition. 12

Coagulation 3. 2. 3 Coagulative separation • While large-sized suspended particles with diameters of up to 10 μm are separable by plain sedimentation and normal filtration, smaller particles with diameter of less than 1 μm cannot be processed mechanically without the use of coagulation, i. e. , coagulative separation. • Furthermore, particles smaller than 0. 001 μm are distributed within the water in molecular form and does not respond to coagulative separation without prior chemical precipitation. • Particulates in the range of 0. 001 to 1 μm are referred to as colloidal particles or simply colloids, and subjected to coagulative separation. 1 nanometer 1 micrometer 1 millimeter 13
![Coagulation Particle sizes and treatment methods Schematic diagram not actual results Chemical treatment andor Coagulation Particle sizes and treatment methods [Schematic diagram, not actual results] Chemical treatment and/or](https://slidetodoc.com/presentation_image_h2/02c472e6f5f9f2ffe0ce4be83cec6474/image-14.jpg)
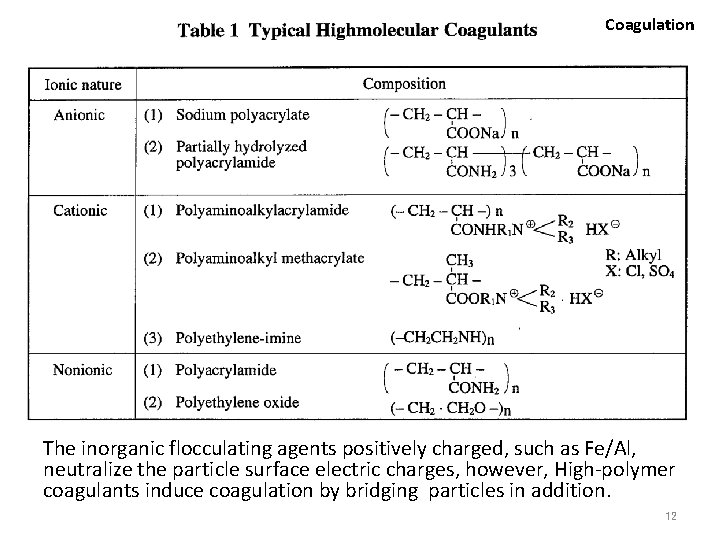
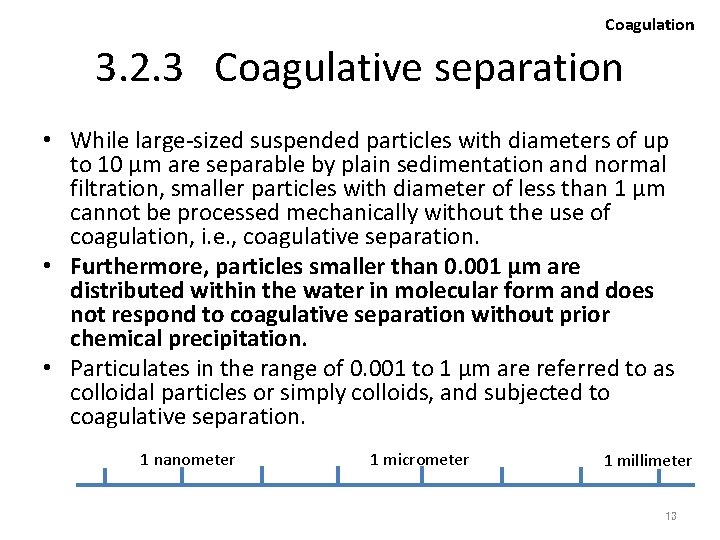
Coagulation Particle sizes and treatment methods [Schematic diagram, not actual results] Chemical treatment and/or Coagulation 10 -10 10 -9 1 nanometer 10 -8 10 -7 Virus 10 -6 1 micrometer Colloid Ion, Molecule Sedimentation 10 -5 10 -4 -3 10 (M) 1 millimeter Suspended solid Bacteria, Small algae Note: The above is just estimated value , not actually measured one. 14

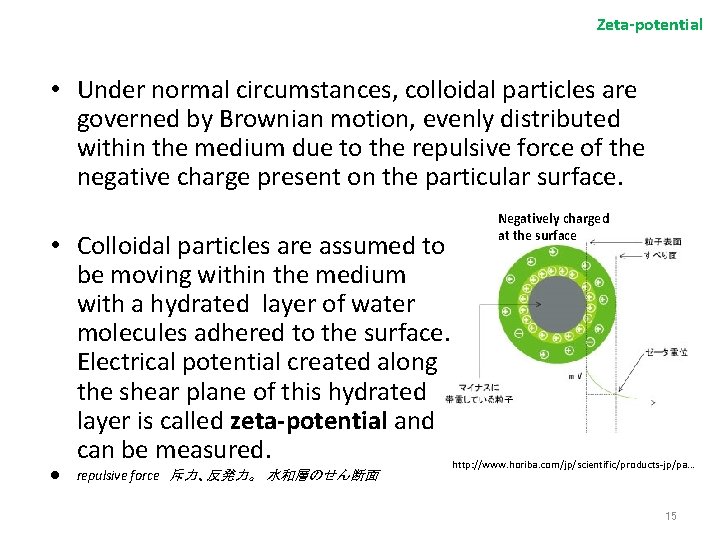
Zeta-potential • Under normal circumstances, colloidal particles are governed by Brownian motion, evenly distributed within the medium due to the repulsive force of the negative charge present on the particular surface. • Colloidal particles are assumed to be moving within the medium with a hydrated layer of water molecules adhered to the surface. Electrical potential created along the shear plane of this hydrated layer is called zeta-potential and can be measured. l repulsive force 斥力、反発力。 水和層のせん断面 Negatively charged at the surface http: //www. horiba. com/jp/scientific/products-jp/pa. . . 15

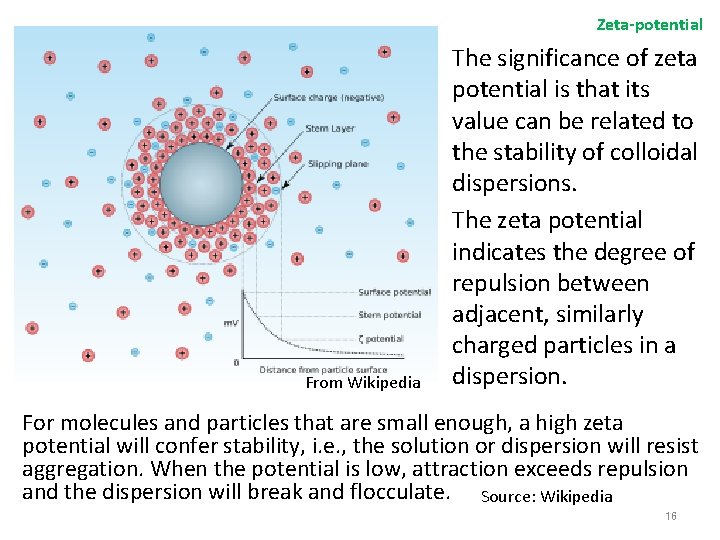
Zeta-potential From Wikipedia The significance of zeta potential is that its value can be related to the stability of colloidal dispersions. The zeta potential indicates the degree of repulsion between adjacent, similarly charged particles in a dispersion. For molecules and particles that are small enough, a high zeta potential will confer stability, i. e. , the solution or dispersion will resist aggregation. When the potential is low, attraction exceeds repulsion and the dispersion will break and flocculate. Source: Wikipedia 16

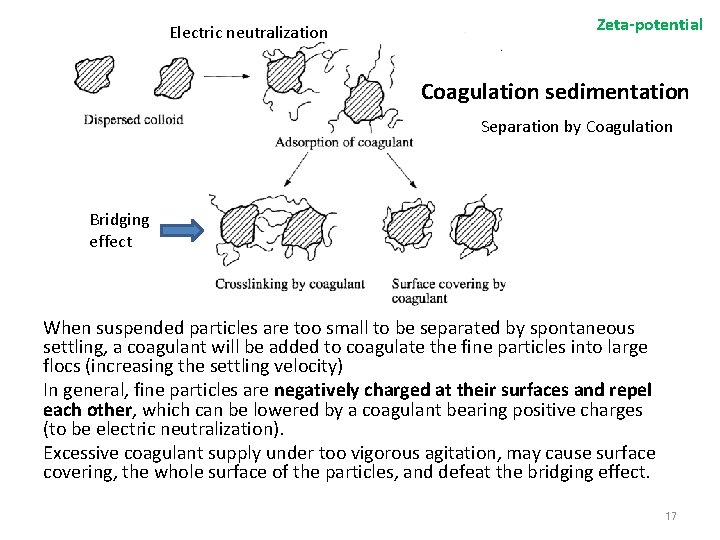
Electric neutralization Zeta-potential Coagulation sedimentation Separation by Coagulation Bridging effect When suspended particles are too small to be separated by spontaneous settling, a coagulant will be added to coagulate the fine particles into large flocs (increasing the settling velocity) In general, fine particles are negatively charged at their surfaces and repel each other, which can be lowered by a coagulant bearing positive charges (to be electric neutralization). Excessive coagulant supply under too vigorous agitation, may cause surface covering, the whole surface of the particles, and defeat the bridging effect. 17

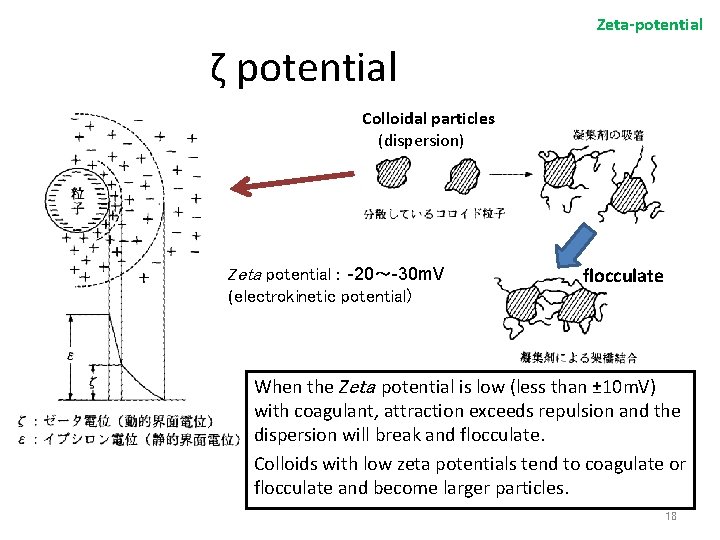
Zeta-potential ζ potential Colloidal particles (dispersion) Zeta potential : -20~-30 m. V (electrokinetic potential) flocculate When the Zeta potential is low (less than ± 10 m. V) with coagulant, attraction exceeds repulsion and the dispersion will break and flocculate. Colloids with low zeta potentials tend to coagulate or flocculate and become larger particles. 18

Coagulation • The zeta-potential generated by colloidal particles evenly distributed within the water is in the range of -20 to -30 m. V. • Upon neutralizing the electric charge of the hydrated layer by adding colloids or ions partaking an opposite (generally positive) charge, thereby adjusting the zetapotential of the suspended particle to within ± 10 m. V, the attractive force acting among the particles (van der Waals attraction) overwhelms the repulsive force of the charged shear plane, thus causing the particles to coagulate. 19

Coagulation • Substances used for such purposes are called flocculating agents. – The agents are generally water soluble with hydrolysis nature, i. e. , hydrolytic cleavaging (break down) properties. • Any metallic salt with the appropriate properties and allowing for the generation of positively charged metal hydroxide colloids will suffice as coagulant, although iron or aluminum salts are used exclusively in the field of water treatment because of their inexpensiveness and non-toxicity. • These metals hydroxides form gelatinous (gel, gelled) sedimentation with porous structure providing for significant surface area. cleavaging 裂け、分割、Gelatinous /dʒəlˈæṭənəs/ゼラチン状/質の; ゲル状の Dispersion分散 hydrolysis加水分解 20

Coagulation • This has a significant influence on the surface charge of the suspended particles, and the gelatinous structure of the sedimentation works favorably toward enhancing the coagulation. • Additional efficacy of physicochemical attachment is also to be expected. • The large agglutinates of suspended particles formed in the process are referred to as “flocs”. 21

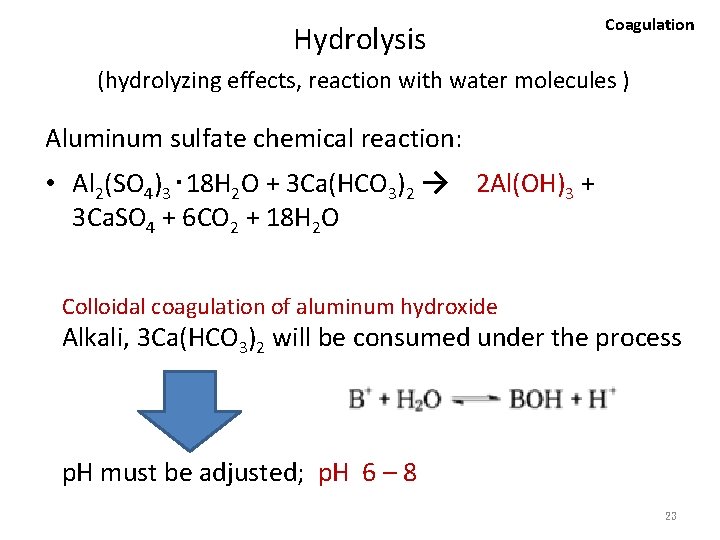
Coagulation 3. 2. 3 (1) Flocculating agents • Aluminum sulfate [Al 2(SO 4)3・ 18 H 2 O] is one of the most commonly used inorganic flocculating agents. – When aluminum sulfate is dissolved in water, the substance hydrolyzes (undergo hydrolysis) as colloidal coagulation of aluminum hydroxide. • The hydroxide takes on many different forms depending on the acidity of the water, but generally expressed as Al(OH)3. Please see next slide. 22

Hydrolysis Coagulation (hydrolyzing effects, reaction with water molecules ) Aluminum sulfate chemical reaction: • Al 2(SO 4)3・ 18 H 2 O + 3 Ca(HCO 3)2 → 2 Al(OH)3 + 3 Ca. SO 4 + 6 CO 2 + 18 H 2 O Colloidal coagulation of aluminum hydroxide Alkali, 3 Ca(HCO 3)2 will be consumed under the process p. H must be adjusted; p. H 6 – 8 23

Coagulation Hydrolytic Cleavage 1 • As indicated in the above slide, the hydrolytic cleavage (hydrolysis) of the flocculating agent consumes the alkali content of the water, changing its p. H level. • There is a most suitable range of p. H for the promotion of the agglutination (conglutination) reaction depending on the type of effluent and flocculating agent used, and the agent may be used with acidic or alkali additives for the adjustment of p. H. • Alkali additives are used more frequently than acidic additives. 24

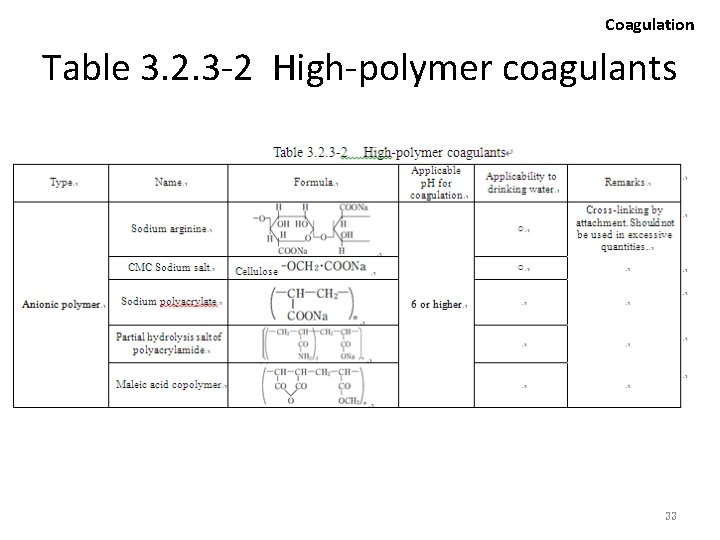
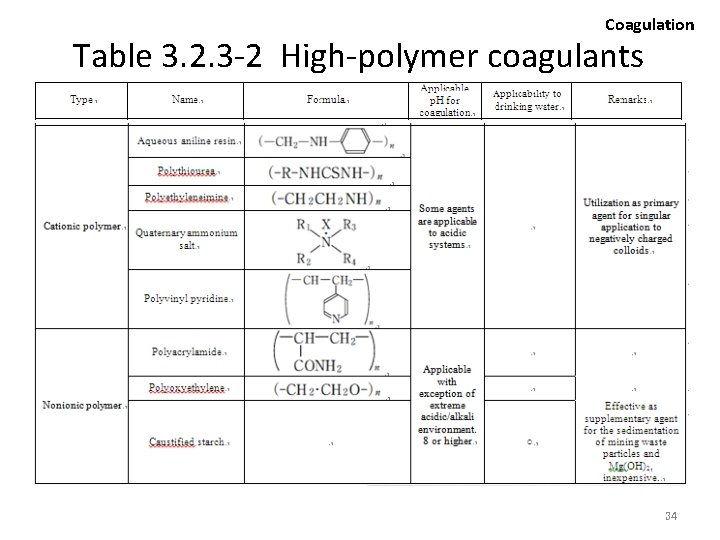
Coagulation Hydrolytic Cleavage 2 • Typical inorganic flocculating agents are provided in table 3. 2. 3 -1. • As the floc produced with inorganic flocculating agents are rather fragile in terms of mechanical strength, the size and sedimentation speed is subject to certain limitations. • The introduction of water soluble polymers with extended chain molecular configuration will enhance the binding power of the floc, consequently yielding larger flocs with a higher rate of sedimentation. • Various types of such high-polymer coagulant are available in Japan, and being utilized in a broad range of effluent treatment. 25

Table 3. 2. 3 -1 Types and properties of inorganic flocculating agents Type Name Aluminum sulfate Aluminum Iron Formula Applicable p. H for coagulation Al 2(SO 4)3・ 18 H 2 O Applicability to drinking water Remarks ○ Most common. Occasionally used with iron salts, also called "Alum". Sodium aluminate Na. Al. O 2 Basic aluminum chloride Polymerized Aln(OH)m. Cl 3 n-m ○ Iron (II) sulfade Iron (III) chloride Iron (III) sulfade Copperas chloride Polysilicato-Iron Fe. SO 4・ 7 H 2 O Fe. Cl 3・ 6 H 2 O Fe 2(SO 4)3・n. H 2 O Fe 2(SO 4)3・Fe. Cl 3 (Si. O 2)n・(Fe 2 O 3) ○ ○ ○ 6 to 8 9 to 11 ○ Said to increase potency when used together with alum. Effective in elimination of chromatic component (color), and capable of reaction without significant alteration of the p. H. May leave iron residue under unsuitable conditions, coloring water. 26

Coagulation • The addition of minor (small) quantities of highpolymer coagulants is sufficient against suspended colloidal particles, successfully yielding larger flocs. • Depending on the charge attributed upon introduction to water, high-polymer coagulants are categorized into cationic, anionic and nonionic agents, with more meticulous differences based on the molecular mass and bifurcation (bunshi-ryo, bunki). • Coagulation effect exhibited by cationic polymer agents are basically assumed to be derived from the neutralization and cross-linking of the negatively charged surface potential of suspended particles. 27

Coagulation • Anionic and nonionic polymers are often used in conjunction with inorganic flocculating agents (ex. Al, Fe), and considered to have their effect on enlarging the floc size by attaching and cross-linking between particles. • The selection of a specific flocculating agent from among many available products for the purification of effluents is an exclusively trial-and-error dependent process. 28

Coagulation • Coagulation sedimentation method sees applications in the elimination of specific categories of contaminants including COD, tainting (color), oil and phosphoric salts as well as being used in the clarification of effluents. • To this end, specific flocculating agents suited for the intended purpose should be selected. • Within this section, descriptions of generic selection criteria are described as follows: 29

Coagulant/ flocculating agent selecting factors (examples) Coagulation Level of treatment Flocs size sedimentation Less chemicals Reducing sludge Userfriendliness No adverse impacts 30

Selecting factors (examples) 1 Coagulation • Goal: To select agents capable of yielding results compliant with the intended purpose of the treatment. • To select agents capable of yielding flocs facilitating the process (in terms of floc size, sedimentation characteristics, etc). • To select agents capable of minimizing the types and quantities of chemicals involved, thereby reducing the running cost of the process. 31

Selecting factors (examples) 2 Coagulation • To select agents capable of reducing the volume of sludge, while concurrently yielding superior properties in the context of sedimentation thickening and dehydration. • To select agents facilitating the transportation, storage, dissolution and addition of chemicals. • To select agents capable of preventing any adverse impacts on the natural environment or the usage of the treated water in the event residues of the agent remain within the water or generated sludge. Typical types of high-polymer coagulants are indicated in table 3. 2. 3 -2. 32

Coagulation Table 3. 2. 3 -2 High-polymer coagulants 33

Coagulation Table 3. 2. 3 -2 High-polymer coagulants 34

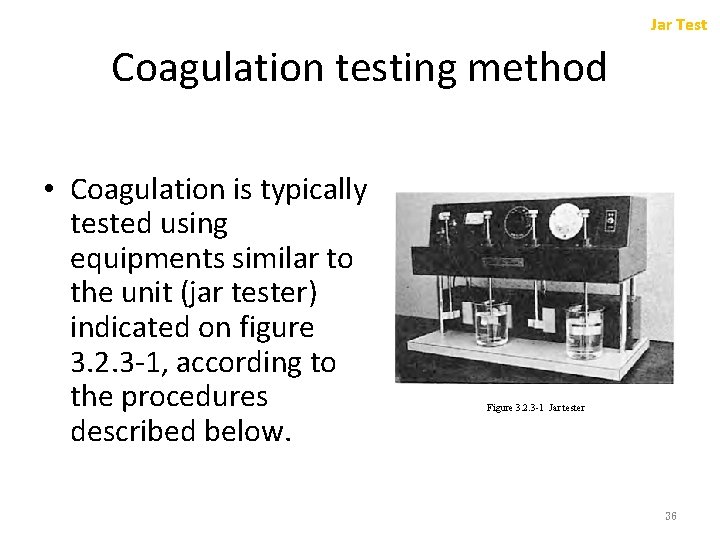
Jar Test 3. 2. 3 (2) Jar Test • Coagulation efficiency varies depending on the kinds and the amount of coagulant added, p. H of the process, the ions coexisting in the system and other factors • The optimum conditions should be decided in advance by a jar test (Beaker test with Jar tester) – Predetermined amounts of sample liquid are taken in individual beakers with different formulations (amount of coagulant, p. H, coexisting ions, etc. ), stirred under the same conditions, and allowed to settle. – Coagulation efficiency is estimated by visual observation or by analyzing the clarified liquor. 35

Jar Test Coagulation testing method • Coagulation is typically tested using equipments similar to the unit (jar tester) indicated on figure 3. 2. 3 -1, according to the procedures described below. Figure 3. 2. 3 -1 Jar tester 36

Jar test process Jar Test • Prepare the necessary quantity of 500 m. L beakers filled with sample of the water to be treated. – 500 m. L of the sample shall be accurately measured into each beaker. – Load the beakers onto the jar tester, lower the agitation axis into the beakers, immersing the agitation blade in the water. • Turn on power switch and activate timer. – The agitation blade shall be set to a rotation of 120 to 150 min-1 and operate. Concurrently introduce the chemicals as indicated. • Promptly introduce the specified amount of chemical (for example, aluminum sulfate solution) into respective beaker using measuring pipettes. – For example, on order to prepare a 1% solution of aluminum sulfate on quantities of 20/40/60/80 mg/L with the specimen quantity of 500 m. L, 1/2/3/4 m. L of the chemical shall be introduced into the beakers respectively. 37

Jar Test Generation of flocs • 1 to 5 minutes after the introduction of the chemical, reduce speed of the agitation blade to 30 to 60 min-1. – The faster rotation used during the initial phase of the test is intended to facilitate the formation of minute flocs by uniform mixing, and the speed is then reduced to allow the created flocs to gradually grow larger. • Stop the agitation after 10 to 20 minutes of gradual rotation, then take out the agitation blade. • Promptly observe the flocs, noting their condition and rate of sedimentation. – The type and quantity of agents capable of creating large flocs with high sedimentation speed while maintaining the clarity of the supernatant (upper clear water) should be selected for use in treating the sample water. 38

Jar Test Jar test • Analyze the water quality of the supernatant. – By conducting an analysis of the water filtered using a filtration paper type 5 -A, the water quality achieved upon executing sand filtration as a post-process to the coagulation can also be estimated. • In evaluating the measurement and design of the treatment system, it is advisable to observe the time required for the flocs to grow to visually identifiable proportions after the rapid agitation phase, as well as whether the generated flocs are easily damaged during the gradual agitation. 39

Jar Test Jar test • Items analyzed in water quality evaluation differ depending on the type of effluent and purpose of treatment, but generally include visual inspection, temperature, turbidity, chromaticity, transparency, p. H and suspended solids. COD, BOD and oil content may also be gauged as needed. 40

3. 2. 3 (3) Components of the coagulation system Coagulation • Coagulating sedimentation is very important process most commonly used in the field of waste water treatment. • In ordinary precipitating separation, the design of the system is based on the sedimentation rate or distribution of the particle diameter of the suspended particle to be separated. • However, the suspended solids subject to the coagulating sedimentation process consist of colloidal distributions incapable of settling by precipitating separation within a limited amount of time. 41

Coagulation • Accordingly, the distribution of particle diameter within the pre-treatment water is not so importance, and the efficiency of the separation process is determined by the capability of the system to coagulate the solids into coarse particles (flocs). time savings • Although ”coagulation” and “sedimentation” are quite different processes, there are systems capable of performing both operations. • Coagulating sedimentation systems must be capable of performing the following procedures: 42

Coagulation ① Introduction of the appropriate flocculating agent • The type of agent to be deployed is not determined by systematic demand, but by the water quality of the suspended substances subjected to the treatment. • In any form of coagulating sedimentation, the types and quantities of flocculating agents are defined solely based on the water quality regardless of the system employed. • As methods to estimate the quantity of agent to be deployed from the result of water quality analysis are yet to be developed, the determination of agent deployment involves a trial-and-error with the jar testing procedure. 43

Coagulation ② Particle concentration • In order for the suspended particles to coagulate into flocs after the surface potential is neutralized by the introduction of flocculating agents, particles need an opportunity to contact each other. • The probability of particle osculation increases as the concentration gets higher. Numerous theoretical formulas are proposed concerning the representation of the rate of coagulation, and can be summarized into the following: 44
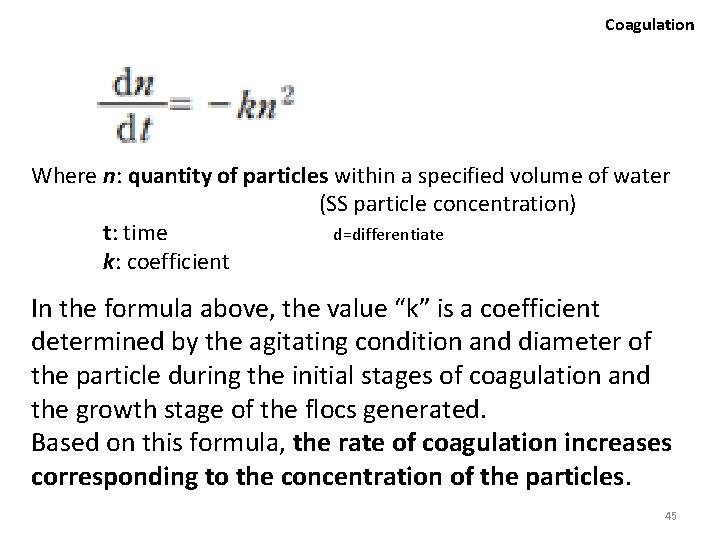
Coagulation Where n: quantity of particles within a specified volume of water (SS particle concentration) d=differentiate t: time k: coefficient In the formula above, the value “k” is a coefficient determined by the agitating condition and diameter of the particle during the initial stages of coagulation and the growth stage of the flocs generated. Based on this formula, the rate of coagulation increases corresponding to the concentration of the particles. 45

③ Appropriate agitation Coagulation • The rate of floc generation relates to the value G signifying the strength of agitation (average velocity gradient of fluids). – Value G is determined from the motivity W per unit volume with the relationship of G = √W/μ (where μ signifies the viscosity coefficient). • If the force of agitation is too excessive, the coagulating floc is broken up by the shear force working in its surroundings and re-disperse. • The optimum condition of agitation is unique to respective coagulating reaction systems. • Such conditions are currently identified empirically (experimentally) as means of theoretically calculating the condition is yet to be developed. 46

Coagulation ④ Sedimenting separation / concentration / sludge removal • The flocs cultured in the coagulation process are then removed from the tank after separation by sedimentation and condensation to an appropriate level of concentration. 47

Coagulation 3. 2. 3(4) Coagulating sedimentation system • All coagulating sedimentation systems are composed of the four factors described in section (3) above. 48

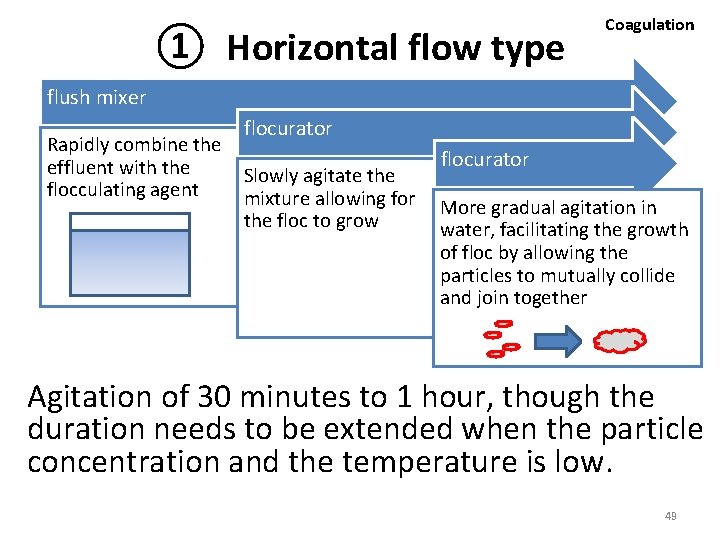
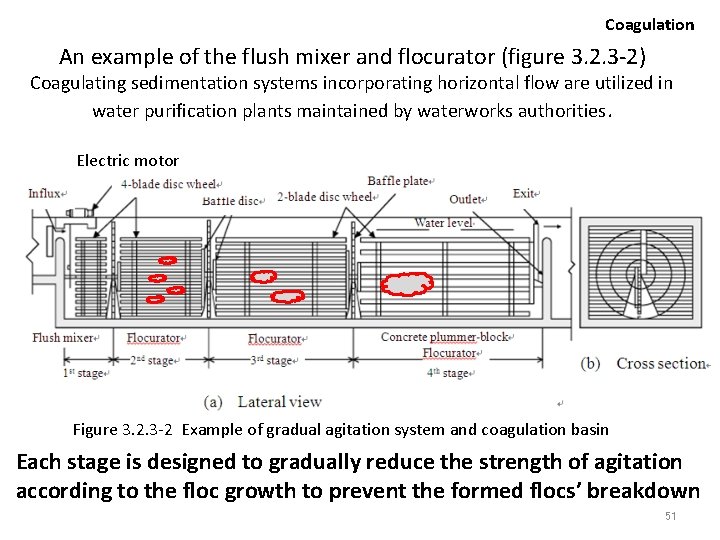
① Horizontal flow type Coagulation flush mixer Rapidly combine the effluent with the flocculating agent flocurator Slowly agitate the mixture allowing for the floc to grow flocurator More gradual agitation in water, facilitating the growth of floc by allowing the particles to mutually collide and join together Agitation of 30 minutes to 1 hour, though the duration needs to be extended when the particle concentration and the temperature is low. 49

Horizontal flow type Coagulation • System consists of a flush mixer, a series of flocurator, and a sedimentation basis for the separation of the enlarged flocs. – The rapid agitation time is about 1 to 5 minutes, with flow speed in the vicinity of the agitation blades empirically set to 1. 5 m/sec (waterworks utility standard). • The flocurator – The particles are attributed with coagulative properties by the flocculating agent. • Each flocurator stage is designed to gradually reduce the strength of agitation 50

Coagulation An example of the flush mixer and flocurator (figure 3. 2. 3 -2) Coagulating sedimentation systems incorporating horizontal flow are utilized in water purification plants maintained by waterworks authorities. Electric motor Figure 3. 2. 3 -2 Example of gradual agitation system and coagulation basin Each stage is designed to gradually reduce the strength of agitation according to the floc growth to prevent the formed flocs’ breakdown 51

Coagulation ② Contact coagulating sedimentation systems • The coagulating reaction can be accelerated by suspending large flocs (referred to as “mother flocs”) in the medium while floc is being produced through the collision of particles. • Various contact coagulating sedimentation systems enable a significant reduction in floc formation time and they can settle the suspended solids very swiftly. • The system is designed in numerous configurations, roughly categorized into three types depending on the method of solid contact facilitation and the mechanism of solid-liquid separation (a) slurry circulation; (b) slurry blanket; and (c) mixed/compound types, further sub-divided by agitation method into mechanical agitation, hydraulic (utilizing water jets or pulsation flow) and pneumatic/air agitation. 52

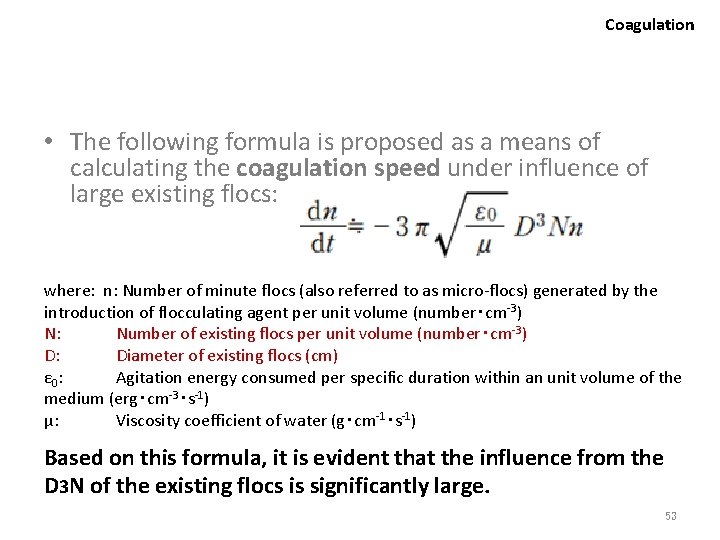
Coagulation • The following formula is proposed as a means of calculating the coagulation speed under influence of large existing flocs: where: n: Number of minute flocs (also referred to as micro-flocs) generated by the introduction of flocculating agent per unit volume (number・cm-3) N: Number of existing flocs per unit volume (number・cm-3) D: Diameter of existing flocs (cm) ε 0: Agitation energy consumed per specific duration within an unit volume of the medium (erg・cm-3・s-1) μ: Viscosity coefficient of water (g・cm-1・s-1) Based on this formula, it is evident that the influence from the D 3 N of the existing flocs is significantly large. 53

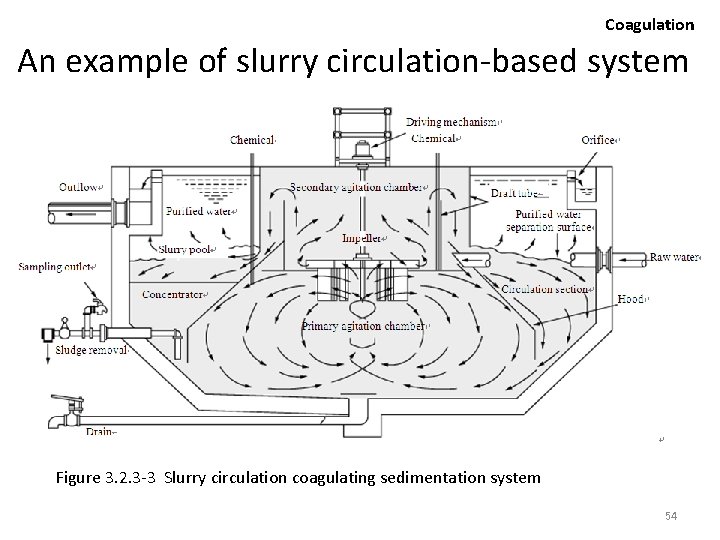
Coagulation An example of slurry circulation-based system Figure 3. 2. 3 -3 Slurry circulation coagulating sedimentation system 54

Coagulation • The raw water is introduced to the primary agitation chamber to be combined with existing flocs and flocculating agents. • The slurry is then agitated by agitation blades, concurrently pumped into the secondary agitation chamber by the movement of the blade, flowing out into the slurry pool through the draft tube. • The solids (floc) are separated from the water within the slurry pool, with the slurry flowing down and circulating back into the primary chamber. • As particles inducted from the raw water are caught in the floc, the particle concentration within the slurry increases with the passage of time. 55

Coagulation • On the context of solid-liquid separation, it is undesirable to increase the density of particle concentration excessively. • Accordingly, the floc is condensed by sedimentation within a concentrator located within the slurry pool and eliminated as necessary to maintain a certain level of concentration. 56

3. 2. 3(5) Applicable scope Coagulation • In coagulating sedimentation, the particle diameter distribution of the source suspended solid is (relatively) irrelevant for the most part, with the sedimentation speed of the floc derived from the coagulation process deciding the efficiency of separation. • Accordingly, coagulation method is applied to colloidal interfusion (mixing, adding) which can not be settled by a natural sedimentation method. • Additionally, the coagulation/sedimentation method effectively separates COD, chromaticity, trace amounts of oil (emulsion) and heavy metal as long as the contaminants are distributed in colloidal form. 57

Phosphoric salt Coagulation • Coagulation also corresponds to the removal of phosphoric salt by utilizing calcium hydroxide or aluminum sulphate (or ferric salt) as the flocculating agent. – In calcium hydroxide method, the p. H of the water is increased to 9. 5 or higher to precipitate the calcium phosphate by chemical reaction prior to coagulation and sedimentation. • In this case, organic polymers or ferric salts are used as the flocculating agent. – In aluminum sulphate method, it is assumed that the aluminum or ferric salts introduced into the water chemically reacts to the phosphorus to form insoluble salts and the coagulation is induces by excess aluminum salts. – Normally, organic polymers or calcium oxide is added to promote the coagulation. – Flocculating agents often added to the aeration tank of the active sludge treatment system to remove the phosphorus contained in organic effluent. 58