Section III Relevance of protein structure and function







- Slides: 58

Section III Relevance of protein structure and function (蛋白质结构与功能的关系)

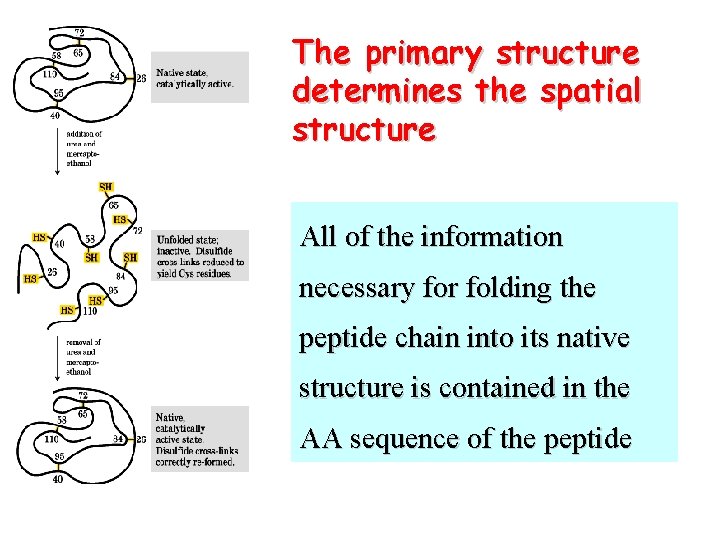
The primary structure determines the spatial structure All of the information necessary for folding the peptide chain into its native structure is contained in the AA sequence of the peptide

The primary structure of a protein: Ø determines its spatial structure Ø is related with evolution of living things Ø has a role in its bioactivity

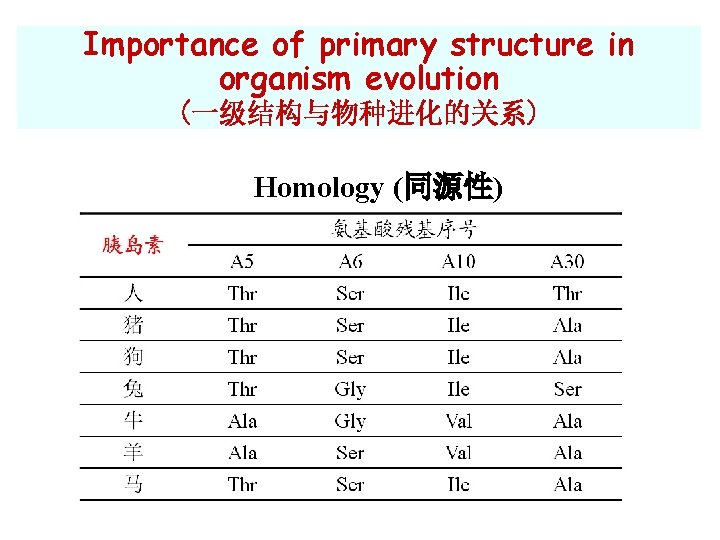
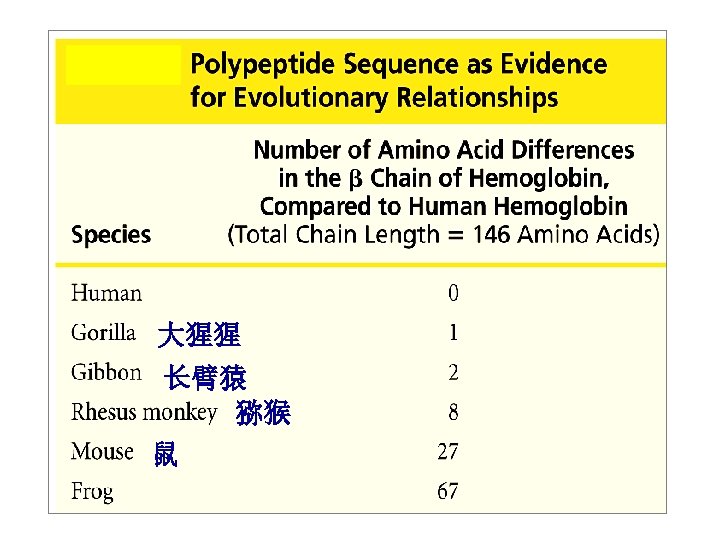
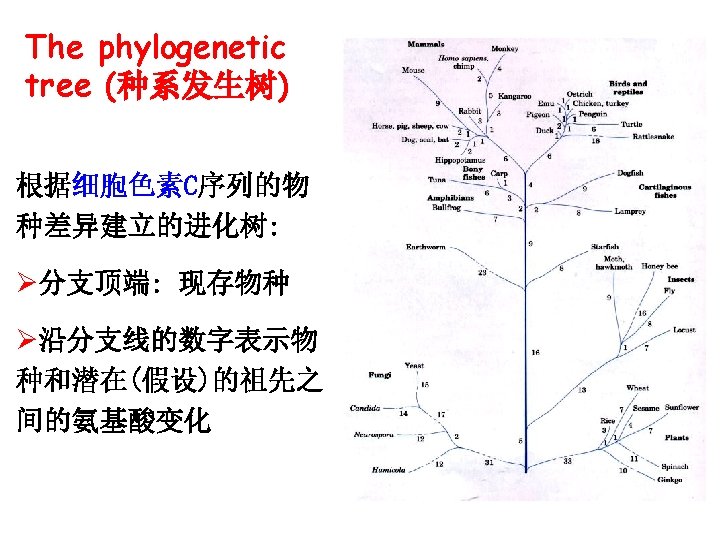
Importance of primary structure in organism evolution (一级结构与物种进化的关系) Homology (同源性)

Primary structure and homology ØHomologous(同源的): proteins with similar AA sequences and functions are said to be homologous ØHomologous proteins from different species imply their evolutionary relatedness



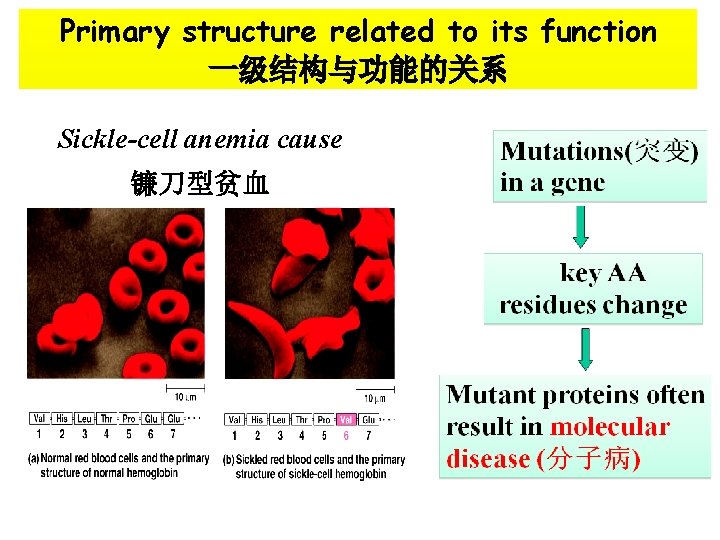
Primary structure related to its function 一级结构与功能的关系 Sickle-cell anemia cause 镰刀型贫血

The changes of key amino acids in its sequence result in function alteration of a protein What is a relationship between the spatial structure of a protein and its function ?

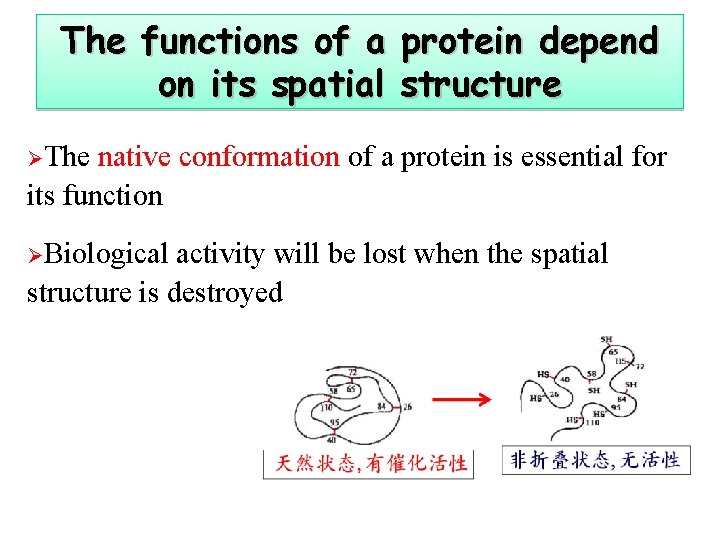
The functions of a protein depend on its spatial structure ØThe native conformation of a protein is essential for its function ØBiological activity will be lost when the spatial structure is destroyed

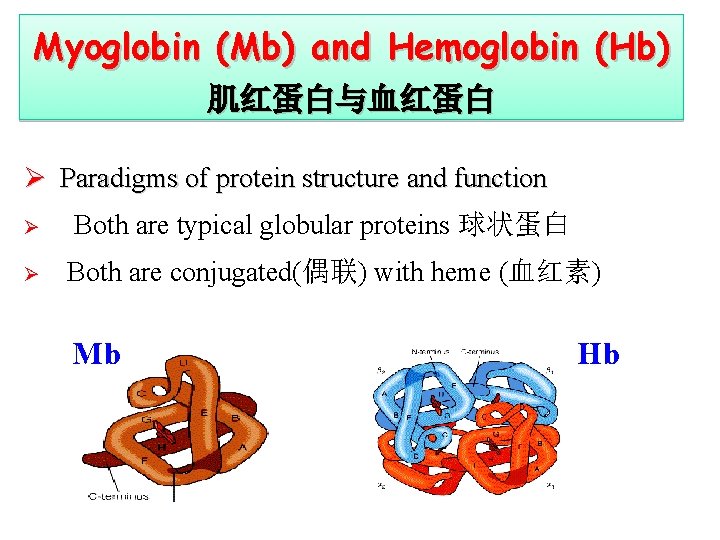
Myoglobin (Mb) and Hemoglobin (Hb) 肌红蛋白与血红蛋白 Ø Paradigms of protein structure and function Ø Ø Both are typical globular proteins 球状蛋白 Both are conjugated(偶联) with heme (血红素) Mb Hb

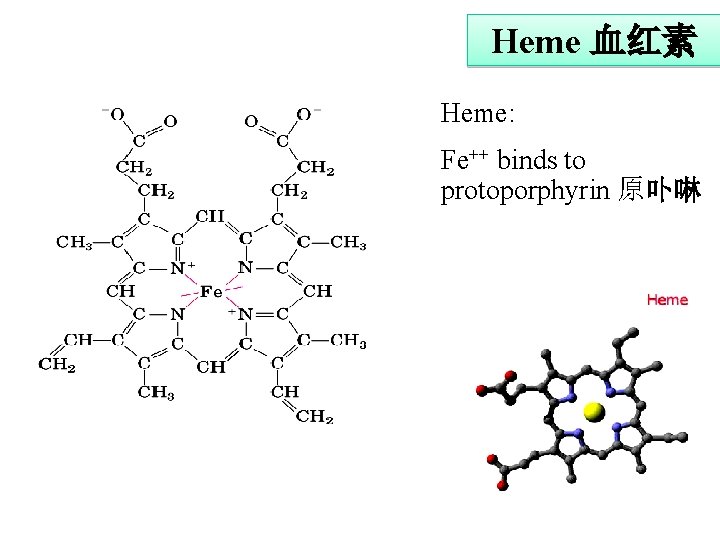
Heme 血红素 Heme: Fe++ binds to protoporphyrin 原卟啉

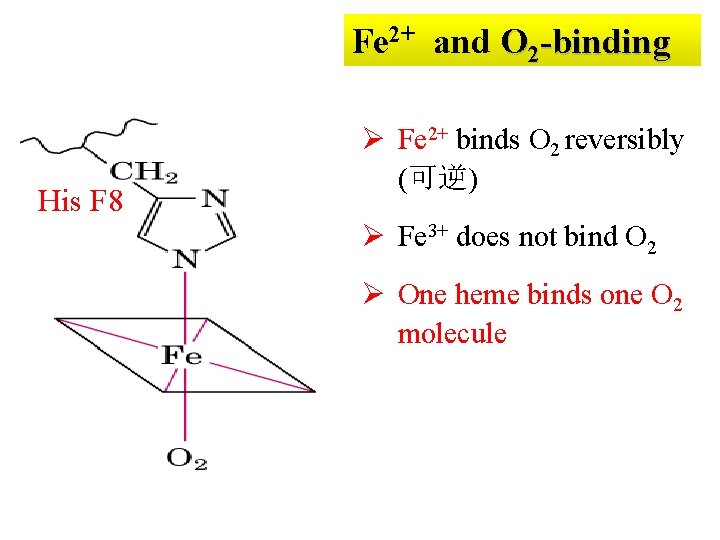
Fe 2+ and O 2 -binding His F 8 Ø Fe 2+ binds O 2 reversibly (可逆) Ø Fe 3+ does not bind O 2 Ø One heme binds one O 2 molecule

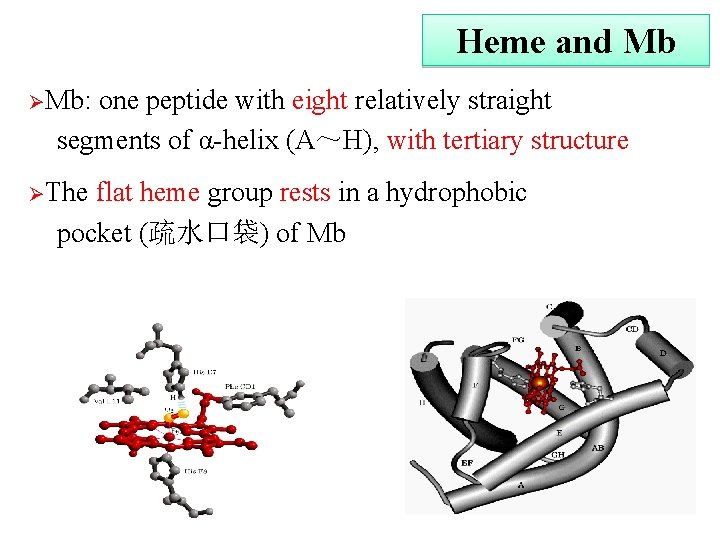
Heme and Mb ØMb: one peptide with eight relatively straight segments of α-helix (A~H), with tertiary structure ØThe flat heme group rests in a hydrophobic pocket (疏水口袋) of Mb

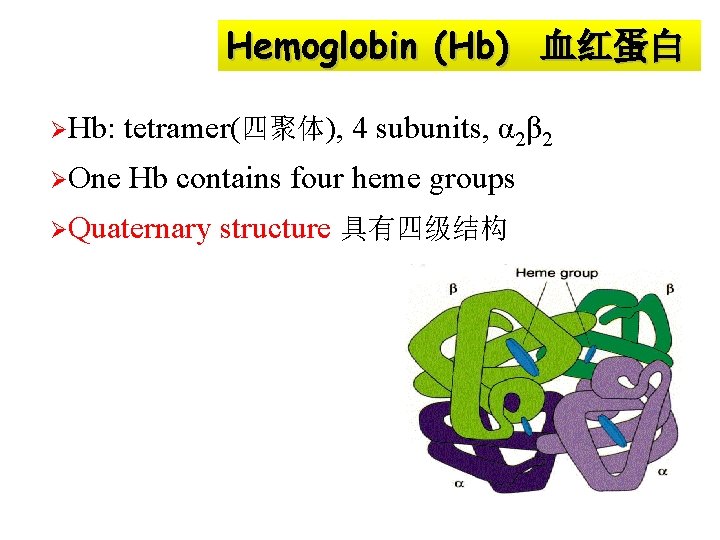
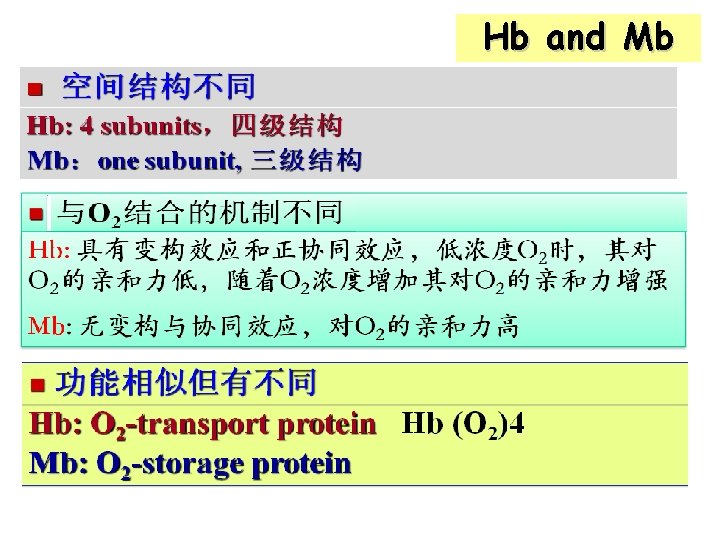
Hemoglobin (Hb) 血红蛋白 ØHb: tetramer(四聚体), 4 subunits, α 2β 2 ØOne Hb contains four heme groups ØQuaternary structure 具有四级结构

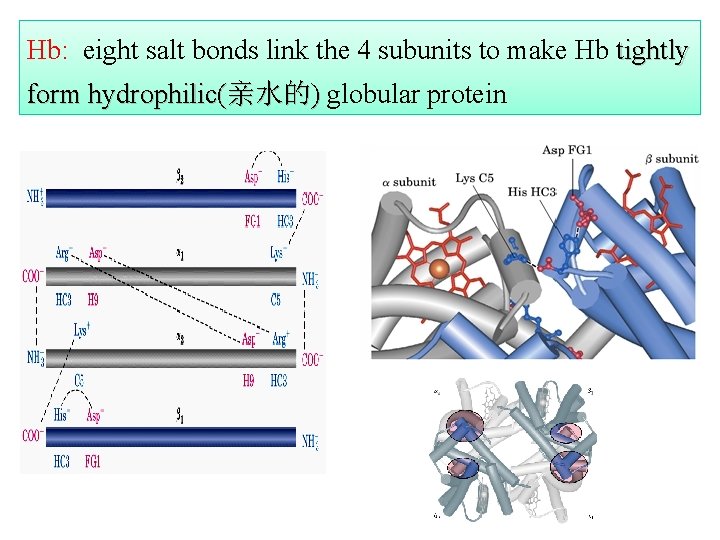
Hb: eight salt bonds link the 4 subunits to make Hb tightly form hydrophilic(亲水的) globular protein

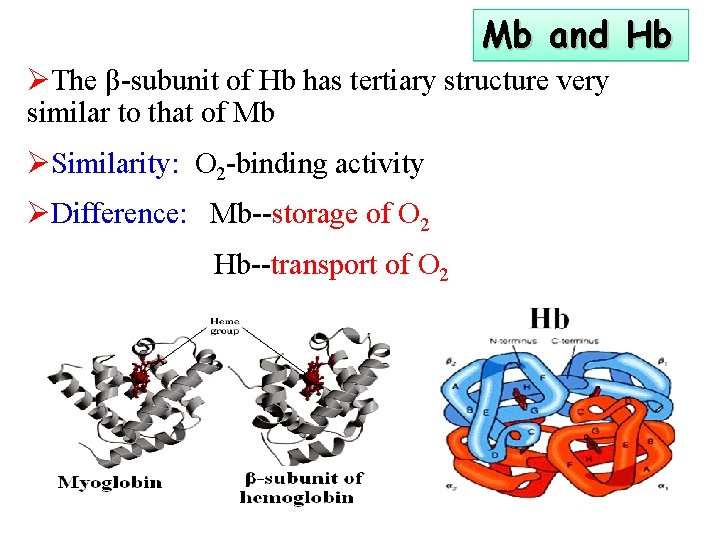
Mb and Hb ØThe β-subunit of Hb has tertiary structure very similar to that of Mb ØSimilarity: O 2 -binding activity ØDifference: Mb--storage of O 2 Hb--transport of O 2

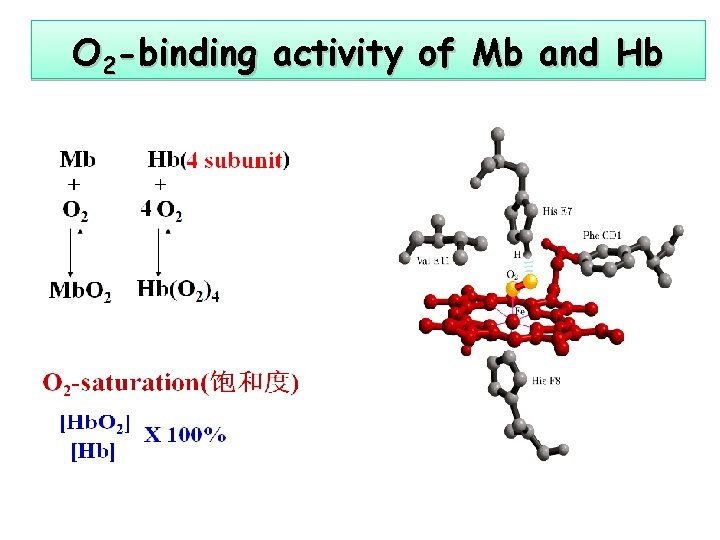
O 2 -binding activity of Mb and Hb

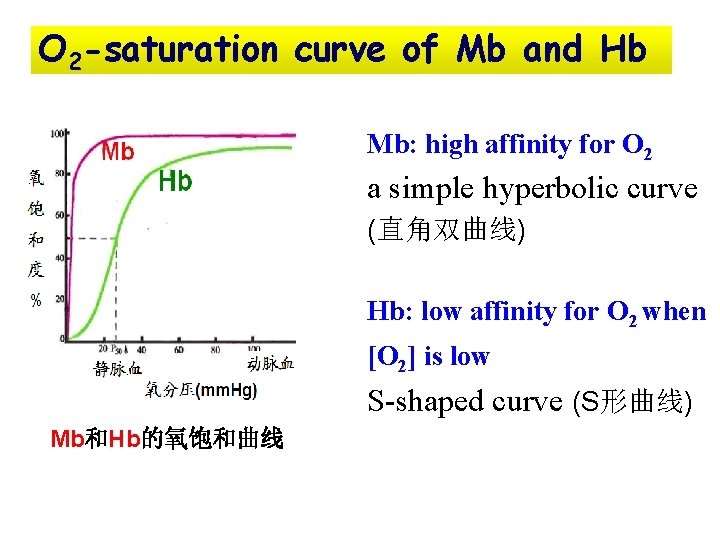
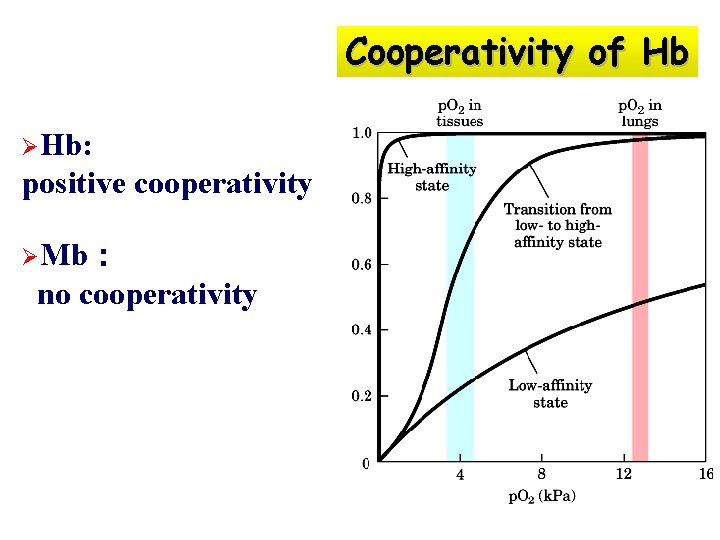
O 2 -saturation curve of Mb and Hb Mb: high affinity for O 2 a simple hyperbolic curve (直角双曲线) Hb: low affinity for O 2 when [O 2] is low S-shaped curve (S形曲线) Mb和Hb的氧饱和曲线

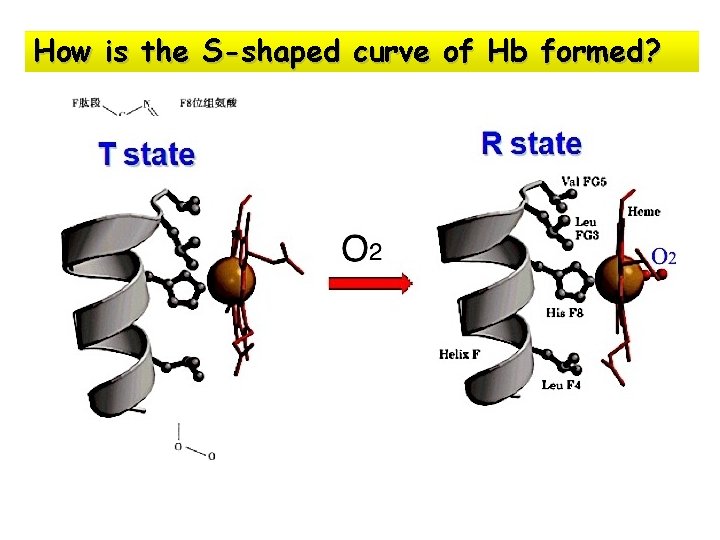
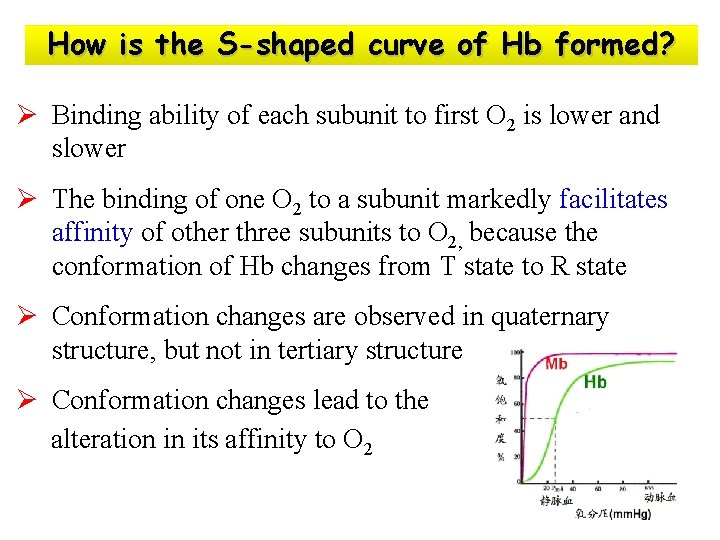
How is the S-shaped curve of Hb formed?

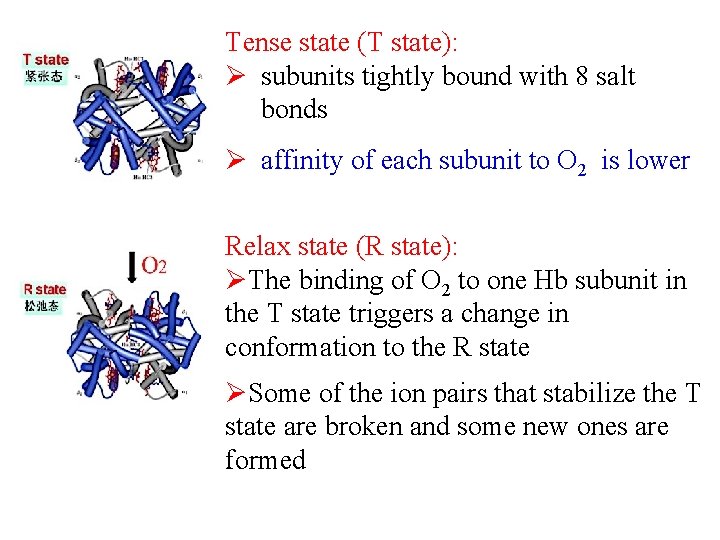
Tense state (T state): Ø subunits tightly bound with 8 salt bonds Ø affinity of each subunit to O 2 is lower Relax state (R state): ØThe binding of O 2 to one Hb subunit in the T state triggers a change in conformation to the R state ØSome of the ion pairs that stabilize the T state are broken and some new ones are formed

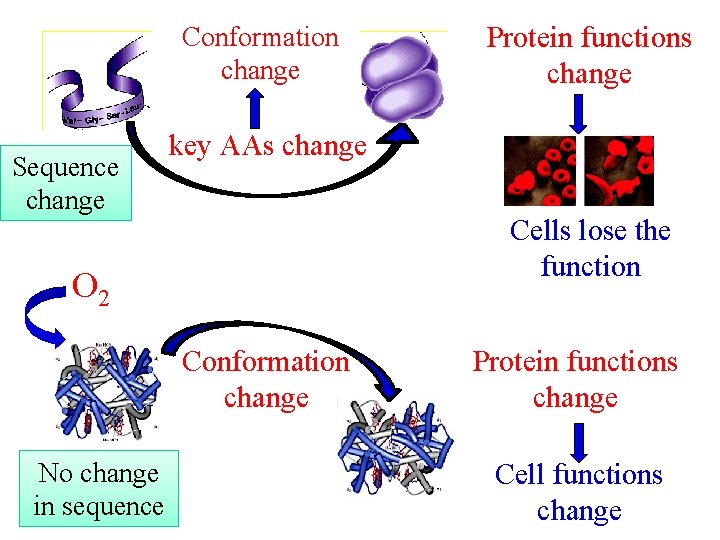
How is the S-shaped curve of Hb formed? Ø Binding ability of each subunit to first O 2 is lower and slower Ø The binding of one O 2 to a subunit markedly facilitates affinity of other three subunits to O 2, because the conformation of Hb changes from T state to R state Ø Conformation changes are observed in quaternary structure, but not in tertiary structure Ø Conformation changes lead to the alteration in its affinity to O 2

Allosteric effect (变构效应) ØLigand molecule (O 2) is binding to a protein (subunit of Hb), which can induce conformation change of the protein and alter its biological activity Ø Allosteric effectors变构效应剂: small molecules e. g: O 2 for Hb

Cooperativity (协同效应) Binding of one ligand molecule to an oligomer protein can change the protein conformation, increase or decrease the probability that further ligand molecules will be bound. ØPositive (正) or negative (负) cooperatvity

Cooperativity of Hb ØHb: positive cooperativity ØMb: no cooperativity

Hb and Mb

Conformation change Sequence change key AAs change Cells lose the function O 2 Conformation change No change in sequence Protein functions change Cell functions change

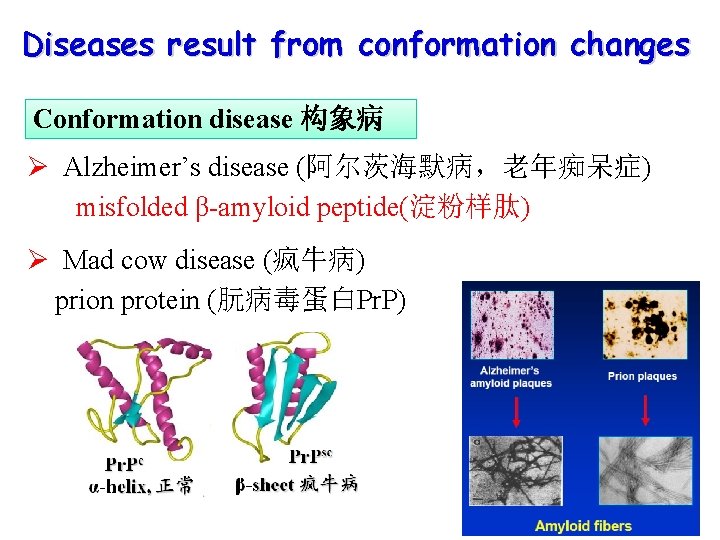
Diseases result from conformation changes Conformation disease 构象病 Ø Alzheimer’s disease (阿尔茨海默病,老年痴呆症) misfolded β-amyloid peptide(淀粉样肽) Ø Mad cow disease (疯牛病) prion protein (朊病毒蛋白Pr. P)

Section IV Properties of proteins 蛋白质的理化性质

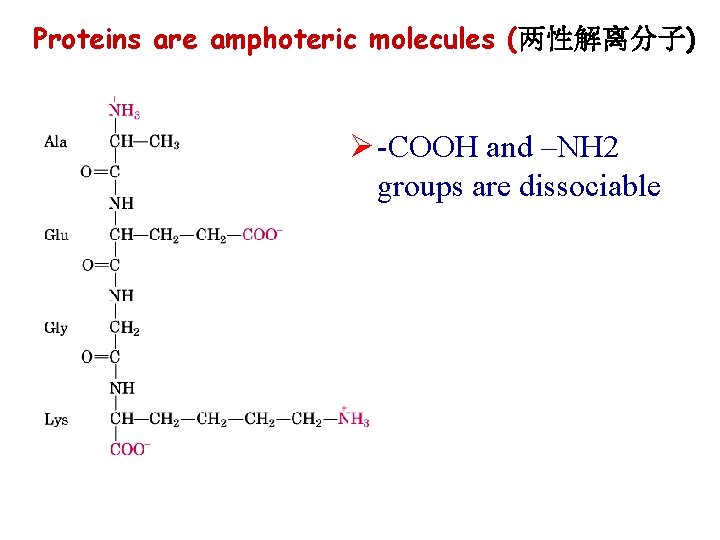
Proteins are amphoteric molecules (两性解离分子) Ø -COOH and –NH 2 groups are dissociable

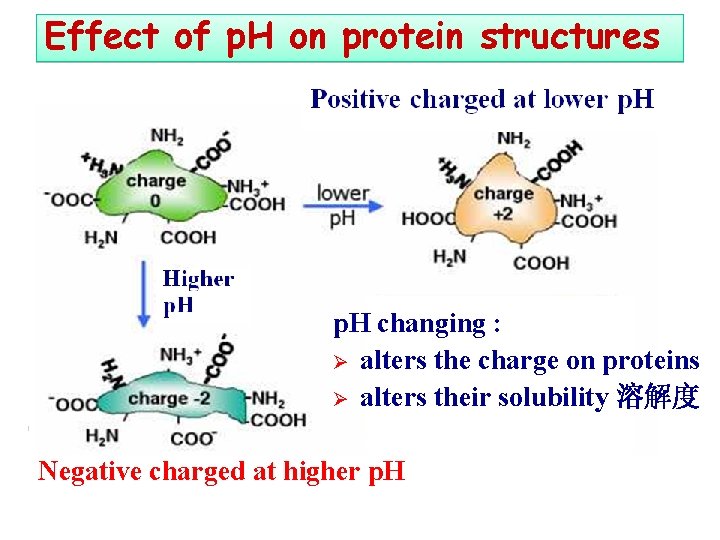
Effect of p. H on protein structures p. H changing : Ø alters the charge on proteins Ø alters their solubility 溶解度 Negative charged at higher p. H

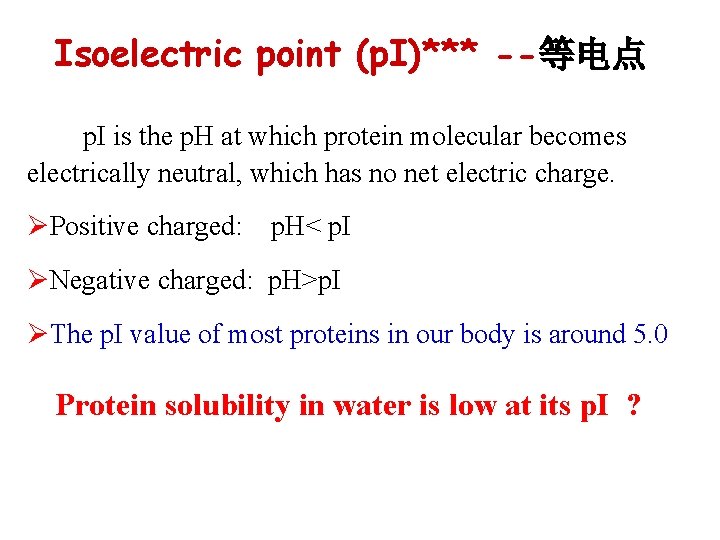
Isoelectric point (p. I)*** --等电点 p. I is the p. H at which protein molecular becomes electrically neutral, which has no net electric charge. ØPositive charged: p. H< p. I ØNegative charged: p. H>p. I ØThe p. I value of most proteins in our body is around 5. 0 Protein solubility in water is low at its p. I ?

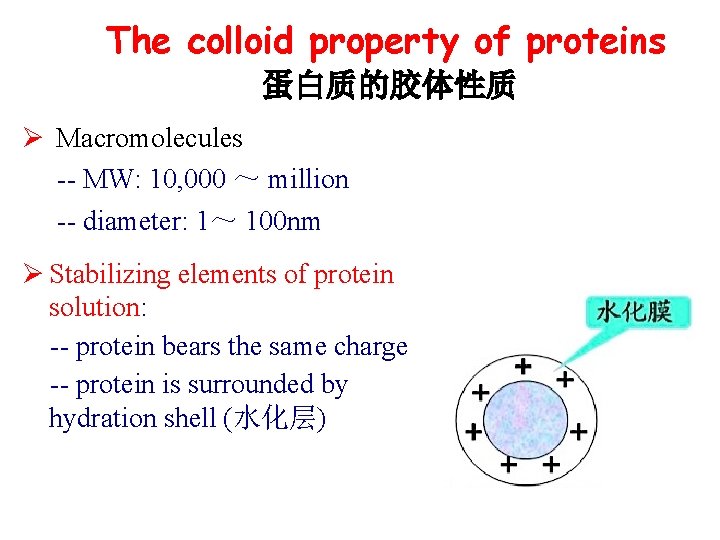
The colloid property of proteins 蛋白质的胶体性质 Ø Macromolecules -- MW: 10, 000 ~ million -- diameter: 1~ 100 nm Ø Stabilizing elements of protein solution: -- protein bears the same charge -- protein is surrounded by hydration shell (水化层)

Denaturation of proteins*** 蛋白质变性 Ø Protein spatial structure is sensitive to denaturing agents (high T, urea (尿素), strong acids or bases, organic solvents, detergents(去污剂), heavy metal ions) Chloroform 氯仿, 三氯甲烷

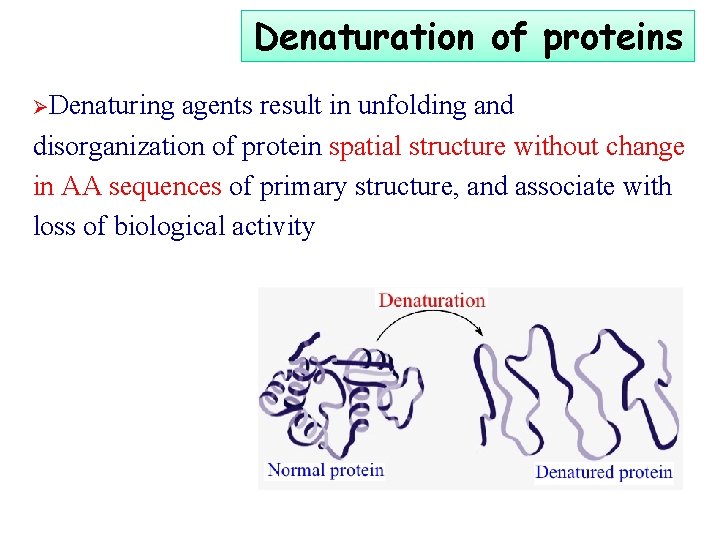
Denaturation of proteins ØDenaturing agents result in unfolding and disorganization of protein spatial structure without change in AA sequences of primary structure, and associate with loss of biological activity

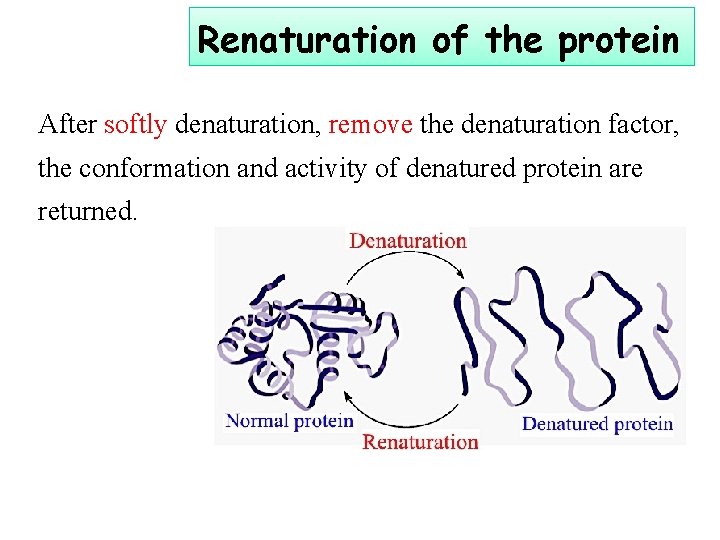
Renaturation of the protein After softly denaturation, remove the denaturation factor, the conformation and activity of denatured protein are returned.

Denatured proteins: Øno peptide bond broken Øbiological activity is partially or completely lost, sensitive to protease effect Øphysical Øusually and chemical properties changed the solubility is reduced

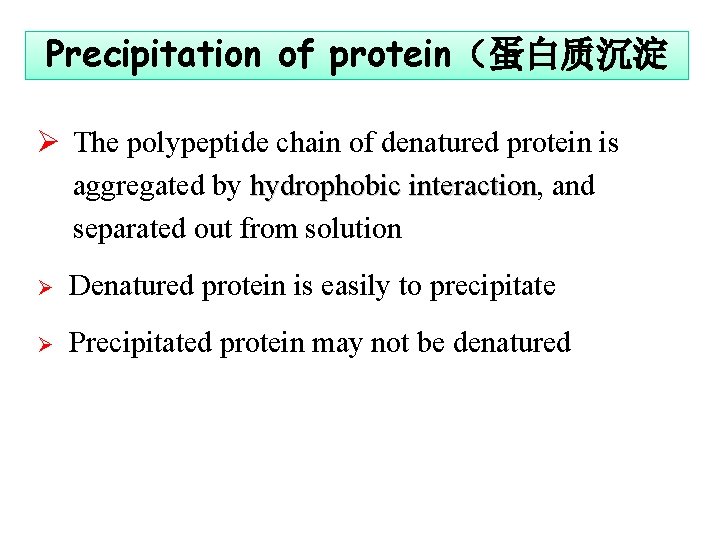
Precipitation of protein(蛋白质沉淀 Ø The polypeptide chain of denatured protein is aggregated by hydrophobic interaction, interaction and separated out from solution Ø Denatured protein is easily to precipitate Ø Precipitated protein may not be denatured

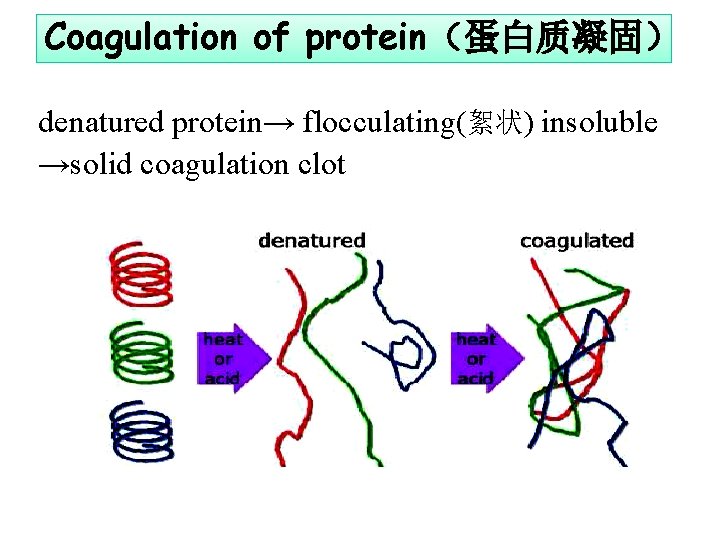
Coagulation of protein(蛋白质凝固) denatured protein→ flocculating(絮状) insoluble →solid coagulation clot

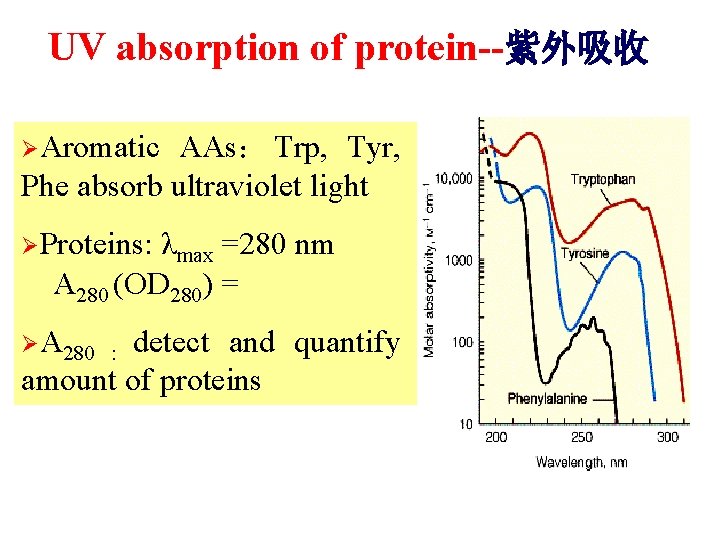
UV absorption of protein--紫外吸收 ØAromatic AAs: Trp, Tyr, Phe absorb ultraviolet light ØProteins: λmax =280 nm A 280 (OD 280) = ØA 280 : detect and quantify amount of proteins

Color test 显色反应 Ø Ninhydrin(茚三酮) Test α-amino acids react with ninhydrin to give a blue-purple product Ø Biuret (双缩脲) reaction All compounds with two or more peptide bonds can react with copper sulfate and produce a violet color product

Section V Isolation & purification & structure analysis of protein 蛋白质的分离纯化与结构分析

Protein isolation and purification Mainly based on physical properties 据物理性质的差异进行分离纯化: Solubility 溶解度 Ø Size 分子大小 Ø Charge 带电性 Ø Density 密度 Ø Biological function 生物功能 Ø


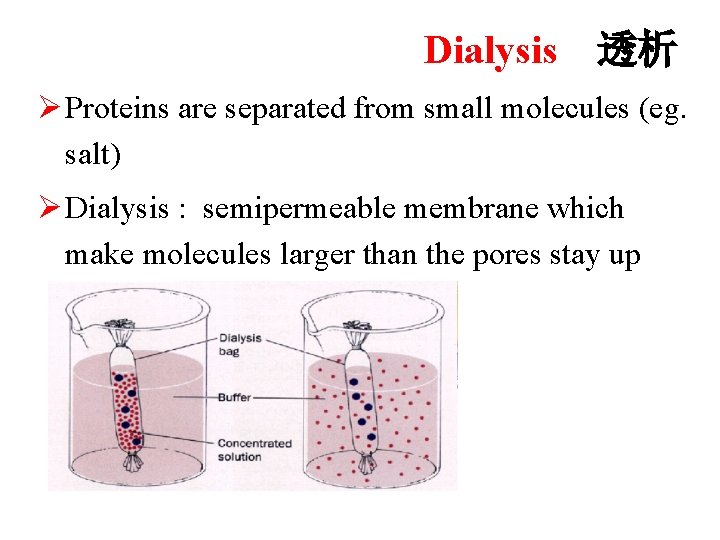
Dialysis 透析 Ø Proteins are separated from small molecules (eg. salt) Ø Dialysis : semipermeable membrane which make molecules larger than the pores stay up

Electrophoresis 电泳 The movement of a charged molecule in an electrical field ØNegatively charged molecules migrate to the anode(正极) ØPositively charged molecules migrate to the cathode(负极)

Electrophoresis Velocity of migration depends upon: Øthe strength of the electric field Øthe net charge on the molecule Øthe size and shape of the molecule Separations usually carried out on a gel ØAgarose gel(琼脂糖凝胶) ØPolyacrylamide gel(聚丙烯酰胺凝胶) 电泳


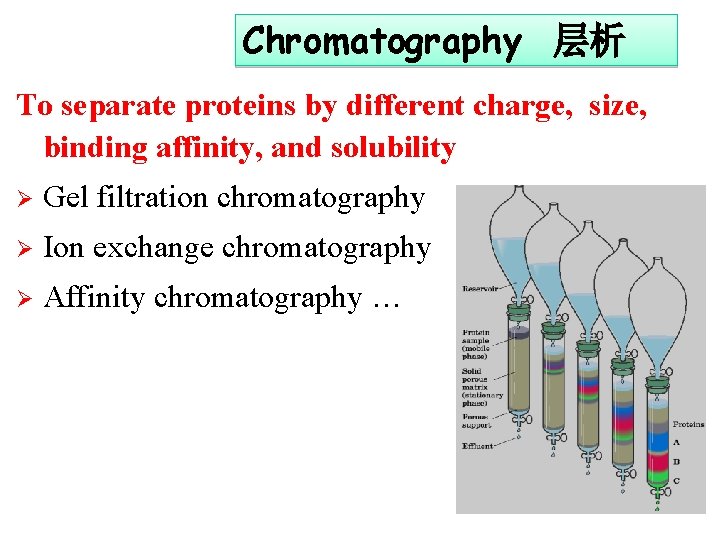
Chromatography 层析 To separate proteins by different charge, size, binding affinity, and solubility Ø Gel filtration chromatography Ø Ion exchange chromatography Ø Affinity chromatography …

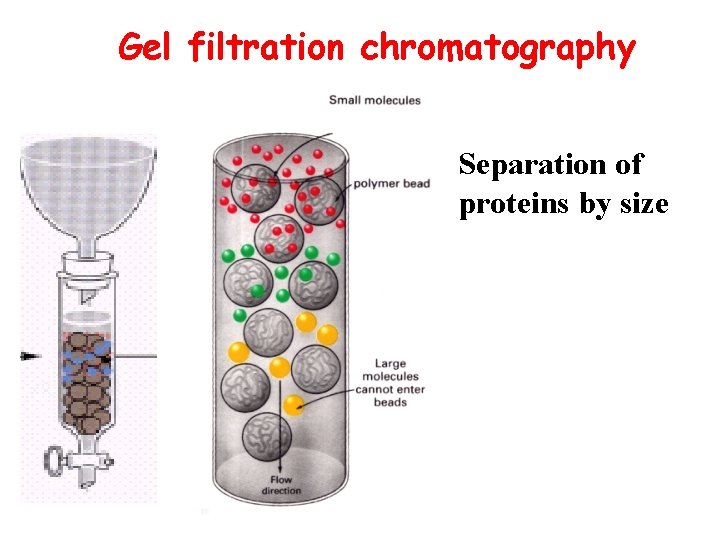
Gel filtration chromatography Separation of proteins by size

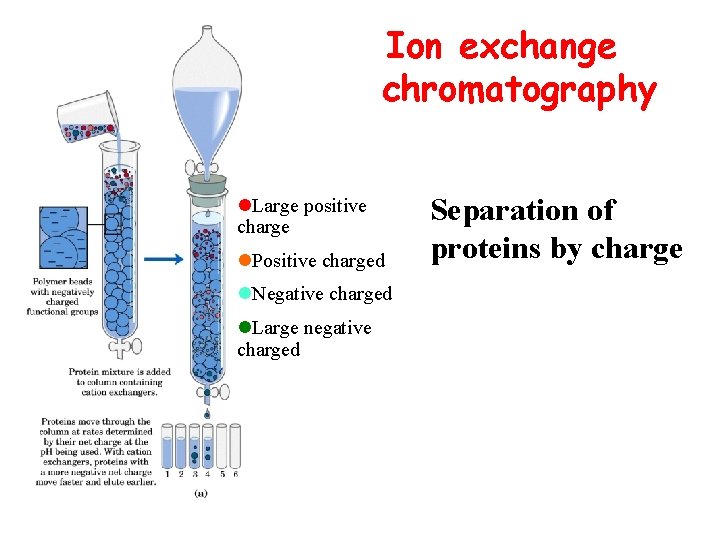
Ion exchange chromatography l. Large positive charge l. Positive charged l. Negative charged l. Large negative charged Separation of proteins by charge

Ultracentrifugation 超速离心 Separation of proteins on the basis of their densities Ø More massive particles always sediment faster Ø Dense particles sediment faster than less dense particles Ø

Primary structure determination自学 AA composition Primary structure analysis– protease hydrolysis separation of peptide segments by HPLC MS analysis


三级结构测定 X射线衍射法(X-ray diffraction)和核磁共 振技术(nuclear magnetic resonance, NMR) 是研究蛋白质三维空间结构最准确的方法。 Crystal preparation X-ray diffraction , NMR


Summary Ø Ø Ø Ø Features of amino acid structure Peptide bond and peptide plane Protein structures: Primary: AA sequence Secondary: α-helix, β-sheet, motif Tertiary and quternary: definition, forces, subunit, domain The primary structure of a protein determines its spatial structure The conformation of a protein is a basis of its function Properties of proteins: denaturation, p. I… Isolation and purification: charge, size, solubility…
